Difference between revisions of "Propylene"
(Propylene) |
|||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | Propylene | + | [[File:Propylene.PNG|thumb|Propylene]] |
| + | [[Propylene]] (or propene) is an alkene (a hydrocarbon containing a carbon-carbon double bond) a colorless gas with a faint petroleum like odor. It is shipped as a liquefied gas under its own vapor pressure. For transportation it may be stenched. Contact with the liquid can cause frostbite. It is easily ignited. The vapors are heavier than air. Any leak can either be liquid or vapor. It can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. It is used to make other chemicals. Can cause explosion.<ref>https://pubchem.ncbi.nlm.nih.gov/compound/Propene#section=Top</ref> | ||
| − | + | ==Production process== | |
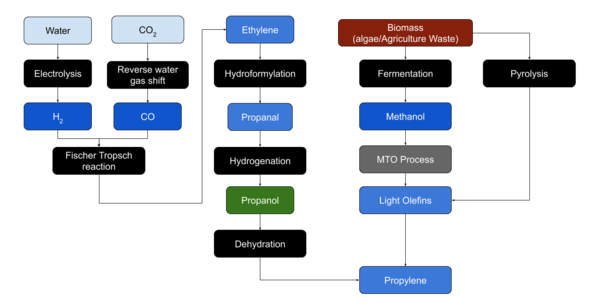
| + | Two production paths may exist on Mars. From [[Water]] and [[CO2]] via the [[Fischer-Tropsch reaction|Fischer Tropsch]] reaction and subsequent processes, or from [[Biomass]]. | ||
| + | |||
| + | ==Uses on Mars== | ||
| + | [[File:Process-Propylene.png|thumb|600x600px|Propylene synthesis]] | ||
| + | Propylene, like many alkenes, are highly desirable as intermediates for petrochemical production. This is a necessary chemical for [[In-situ resource utilization|chemical production on Mars (In-situ resource utilization)]]. Two important uses are in the production of [[Synthetic materials|polypropylene]] (via polymerization) and butanol (via hydroformylation). | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | * [[w:Propene|Wikipedia article]] | ||
| + | <references /> | ||
Latest revision as of 08:59, 27 November 2020
Propylene (or propene) is an alkene (a hydrocarbon containing a carbon-carbon double bond) a colorless gas with a faint petroleum like odor. It is shipped as a liquefied gas under its own vapor pressure. For transportation it may be stenched. Contact with the liquid can cause frostbite. It is easily ignited. The vapors are heavier than air. Any leak can either be liquid or vapor. It can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. It is used to make other chemicals. Can cause explosion.[1]
Production process
Two production paths may exist on Mars. From Water and CO2 via the Fischer Tropsch reaction and subsequent processes, or from Biomass.
Uses on Mars
Propylene, like many alkenes, are highly desirable as intermediates for petrochemical production. This is a necessary chemical for chemical production on Mars (In-situ resource utilization). Two important uses are in the production of polypropylene (via polymerization) and butanol (via hydroformylation).