Difference between revisions of "Thorium"
m |
m (→Carbonyl chemical reactions: Added link.) |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
|abundance=}}Thorium, ''[[Elements on Mars|Periodic table]] Th'', is present on [[Mars]], however, its surface concentration seems to be lower than on Earth.<ref name=":12">Map of Martian Thorium at Mid-Latitudes, JPL '' Map of Martian Thorium at Mid-Latitudes '', https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.</ref> Thorium can be used to produce fuel for [[Nuclear power|nuclear reactors]] on Mars, including [[LFTR|Liquid Fluoride Thorium Reactors]], [[Nuclear_thermal_propulsion|nuclear thermal propulsion]] and [[Ion_thruster#Pulsed_Fission_Fusion_.28PuFF.29|nuclear pulsed propulsion]]. | |abundance=}}Thorium, ''[[Elements on Mars|Periodic table]] Th'', is present on [[Mars]], however, its surface concentration seems to be lower than on Earth.<ref name=":12">Map of Martian Thorium at Mid-Latitudes, JPL '' Map of Martian Thorium at Mid-Latitudes '', https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.</ref> Thorium can be used to produce fuel for [[Nuclear power|nuclear reactors]] on Mars, including [[LFTR|Liquid Fluoride Thorium Reactors]], [[Nuclear_thermal_propulsion|nuclear thermal propulsion]] and [[Ion_thruster#Pulsed_Fission_Fusion_.28PuFF.29|nuclear pulsed propulsion]]. | ||
| − | Note that Thorium has a very long half life of 14 billion years, so the majority of the thorium that existed when the Earth formed is still here ( | + | Note that Thorium has a very long half life of 14 billion years, so the majority of the thorium that existed when the Earth and Mars were formed is still here (the planets are is 4.5 billion years old). Thorium is not fissile, but it is fertile. In other words, thorium does not spontaneously fission, but with neutron bombardment, it will transform into fissionable U233. |
==Concentration of thorium== | ==Concentration of thorium== | ||
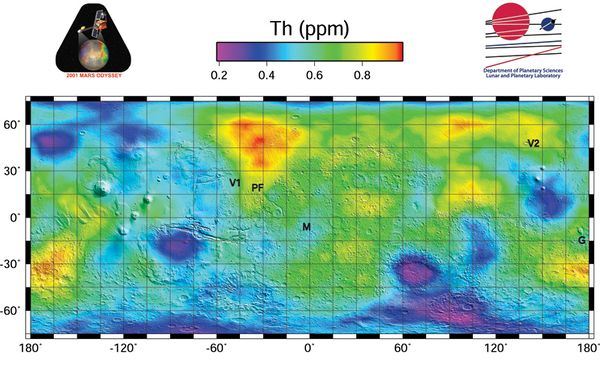
| − | The average surface concentration is 0,6 ppm, or about ten times lower than Earth' average abundance of 6 ppm, with some high concentration areas of about 1 ppm <ref>https://en.wikipedia.org/wiki/Occurrence_of_thorium</ref>. See map. Martian basalts may have concentrations of 5 ppm(), similar to the basalts of Earth. Monazite (a phosphate mineral that also includes rare Earth elements) mines on Earth can have a concentration | + | The average surface concentration is 0,6 ppm, or about ten times lower than Earth' average abundance of 6 ppm, with some high concentration areas of about 1 ppm <ref>https://en.wikipedia.org/wiki/Occurrence_of_thorium</ref>. See map. Martian basalts may have concentrations of 5 ppm(), similar to the basalts of Earth. Monazite (a phosphate mineral that also includes rare Earth elements) mines on Earth can have a concentration from 500ppm to 25,000 ppm of Thorium. Naturally concentrated deposits are welcome, but are not needed to make Thorium economical on Mars.<ref>https://aip.scitation.org/doi/pdf/10.1063/1.4972913</ref> <ref>https://www.youtube.com/watch?v=lxwF93wnRQo</ref> Mines producing other metals could be utilized, many which produce a waste stream enriched in thorium.<ref>https://trace.tennessee.edu/cgi/viewcontent.cgi?article=3397&context=utk_chanhonoproj</ref><ref>https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes</ref> |
This page: [[Radioactive Rarity on Mars]] discusses the apparent rarity of radioactive elements on Mars. | This page: [[Radioactive Rarity on Mars]] discusses the apparent rarity of radioactive elements on Mars. | ||
[[File:Th 040305 NG 5x5 SmB10 016 EQ75 with2Logos web.jpg|thumb|600x600px|Thorium surface concentration on Mars in PPM.]] | [[File:Th 040305 NG 5x5 SmB10 016 EQ75 with2Logos web.jpg|thumb|600x600px|Thorium surface concentration on Mars in PPM.]] | ||
| + | |||
| + | <span style="color: blue><sup>Citation Needed</sup></span> | ||
==How to breed Th232 into U233== | ==How to breed Th232 into U233== | ||
| Line 24: | Line 26: | ||
If you don't understand the above paragraph, the following page on [[Isotopes]] will help. | If you don't understand the above paragraph, the following page on [[Isotopes]] will help. | ||
| − | Note that as the U233 is bred, a small amount of U232 is also created. This decays releasing powerful gamma rays twice in its early decay chain, which makes U233 | + | Note that as the U233 is bred, a small amount of U232 is also created. This decays releasing powerful gamma rays twice in its early decay chain, which is bad and good. (Bad in that the reactor core is even MORE radioactive, good in that in makes U233 less attractive for atom bomb making.) |
| + | |||
| + | A molten salt reactor called a [[LFTR]] is designed to breed Thorium into U233. | ||
==Cost to refine & use thorium== | ==Cost to refine & use thorium== | ||
| Line 31: | Line 35: | ||
Thorium is about as rare on Earth as lead, and lead is extracted at $2/kg. However, thorium is a nuisance by-product of Rare-Earth Elements (REE) mining, with no commercial use, so it is currently very cheap. (Lead is extracted from sulphides which are cheaper to refine than thorium which is usually found in oxides, so thorium would likely be from 4 to 6 times more expensive to reduce from its ores.) Lead is about 10 parts per million in the Earth's crust. Thorium is about 6 ppm. | Thorium is about as rare on Earth as lead, and lead is extracted at $2/kg. However, thorium is a nuisance by-product of Rare-Earth Elements (REE) mining, with no commercial use, so it is currently very cheap. (Lead is extracted from sulphides which are cheaper to refine than thorium which is usually found in oxides, so thorium would likely be from 4 to 6 times more expensive to reduce from its ores.) Lead is about 10 parts per million in the Earth's crust. Thorium is about 6 ppm. | ||
| − | However, thorium ores | + | However, thorium ores have much higher concentrations: thorianite (rare) is 12% ThO (plus a good deal of useful uranium); monazite is 2.5% Th (25,000 ppm); allanite is 0.1 to 2% Th (1,000 to 20,000 ppm); zircon can have up to 0.4% Th (4,000 ppm).<ref>https://en.wikipedia.org/wiki/Occurrence_of_thorium</ref> |
If we assume that thorium extraction is 6 times the cost of extracting lead, times 1/60% (since Th is slightly rarer than lead) we get a cost of $20 / kg. (Note: Google says that you can buy thorium at $30/kg. Perhaps if thorium was mined in as large quantities as lead, this price would drop. I'll use the google value from this point forward.) | If we assume that thorium extraction is 6 times the cost of extracting lead, times 1/60% (since Th is slightly rarer than lead) we get a cost of $20 / kg. (Note: Google says that you can buy thorium at $30/kg. Perhaps if thorium was mined in as large quantities as lead, this price would drop. I'll use the google value from this point forward.) | ||
===Value of thorium's energy=== | ===Value of thorium's energy=== | ||
| − | The specific energy of thorium is 79,420,000 megaJoules / kg. (This is the equivalent of 12,977 barrels of oil - a huge amount of energy.) In the USA an average home uses 10,600 kWh per year. <ref>https://www.eia.gov/tools/faqs/faq.php?id=97&t=3</ref>. 1 kiloWatt hour = 3.6e^6 Joules, so 10,600 kWh = 38,160 megaJoules. Dividing this into the specific energy of 1 kg of Thorium, 1 kg of thorium (if completely fissioned, and converted to electrical power at 50% efficiency) would power 1,040 homes for a year. Rounding off, call it a town of 1,000 houses. | + | The specific energy of thorium is 79,420,000 megaJoules / kg.<ref>https://en.wikipedia.org/wiki/Energy_density</ref> (This is the equivalent of 12,977 barrels of oil - a huge amount of energy.) In the USA an average home uses 10,600 kWh per year. <ref>https://www.eia.gov/tools/faqs/faq.php?id=97&t=3</ref>. 1 kiloWatt hour = 3.6e^6 Joules, so 10,600 kWh = 38,160 megaJoules. Dividing this into the specific energy of 1 kg of Thorium, 1 kg of thorium (if completely fissioned, and converted to electrical power at 50% efficiency) would power 1,040 homes for a year. Rounding off, call it a town of 1,000 houses. |
As of 2022, the average cost of electricity in the USA is $0.142 per kWh. Multiplying this by 10,600 kWh / home * 1,000 homes = $1,505,000. Using the Google price of thorium at $30 / kg, it can be seen that the fuel price is insignificant compared to the value of the power. (If you want to round off a little, some easy to remember values are: 1 kg of Th will produce ~1 MW (electric) in a year, which is enough to power 1,000 homes, which is worth $1.5 M.) | As of 2022, the average cost of electricity in the USA is $0.142 per kWh. Multiplying this by 10,600 kWh / home * 1,000 homes = $1,505,000. Using the Google price of thorium at $30 / kg, it can be seen that the fuel price is insignificant compared to the value of the power. (If you want to round off a little, some easy to remember values are: 1 kg of Th will produce ~1 MW (electric) in a year, which is enough to power 1,000 homes, which is worth $1.5 M.) | ||
| Line 51: | Line 55: | ||
* The next assumption that the thorium would burn up 100% is more suspect. Typical fission power plants burn only 0.5% of their fuel before it is 'spent' and sent to long term waste. However, current light water reactors were designed 70 years ago, and are WILDLY inefficient. They use SOLID fuel oxides, clad in zirconium alloy cladding. As the U235 fissions, the fuel elements swell and crack, releasing fission products (especially Xenon gas which is the worse neutron poison). The fuel cylinders swell which puts pressure on the Zr cladding. The zirconium cladding becomes brittle from neutron bombardment. So before they break, the fuel rod is removed (say after 17 months), and a new one is put in its place. The old fuel rods should be dissolved in acid, the fission products discarded, the 99.5% unburnt uranium recovered, converted back into a solid oxide and put back in a new fuel rod. But the fission products are wildly, dangerously radioactive, and this chemical processing is expensive (especially considering the radioactivity). Fresh fuel is so cheap, that it is more profitable to discard the used fuel rods, even tho more than 99% of the uranium has not fissioned. | * The next assumption that the thorium would burn up 100% is more suspect. Typical fission power plants burn only 0.5% of their fuel before it is 'spent' and sent to long term waste. However, current light water reactors were designed 70 years ago, and are WILDLY inefficient. They use SOLID fuel oxides, clad in zirconium alloy cladding. As the U235 fissions, the fuel elements swell and crack, releasing fission products (especially Xenon gas which is the worse neutron poison). The fuel cylinders swell which puts pressure on the Zr cladding. The zirconium cladding becomes brittle from neutron bombardment. So before they break, the fuel rod is removed (say after 17 months), and a new one is put in its place. The old fuel rods should be dissolved in acid, the fission products discarded, the 99.5% unburnt uranium recovered, converted back into a solid oxide and put back in a new fuel rod. But the fission products are wildly, dangerously radioactive, and this chemical processing is expensive (especially considering the radioactivity). Fresh fuel is so cheap, that it is more profitable to discard the used fuel rods, even tho more than 99% of the uranium has not fissioned. | ||
| − | A LFTR burning thorium has a different philosophy. The unburnt fuel STAYS in the reactor until it 'burns'. The best place for these heavy nucleotides, is ''inside'' the reactor. Ideally, the only thing leaving the reactor is heat and fission products. The molten salt is a LIQUID so you can do chemical processes on it directly. Helium gas can be bubbled thru the working salt to pull out gases (such as xenon, radon, and tritium). Some elements will plate out on cool surfaces. (Tiny quantities of platinum, gold, iridium, etc. can be gained this way.) As for metals that will dissolve in the molten salt, they can be removed with some effort which requires many successive chemical steps. Originally it was intended to remove them constantly, but recently the idea is to let these products build up for a couple years, then remove the salt to a specialized plant to remove these wastes. While the wastes are in the reactor, they will take up a small amount of volume, reducing the reactor's efficiency slightly. (This waste processing is the least developed part of LFTR. It has been designed on paper, but little practical work has been done.) | + | A LFTR burning thorium has a different philosophy. The unburnt fuel STAYS in the reactor until it 'burns'. The best place for these heavy nucleotides, is ''inside'' the reactor. Ideally, the only thing leaving the reactor is heat and fission products. The molten salt is a LIQUID so you can do chemical processes on it directly. Helium gas can be bubbled thru the working salt to pull out gases (such as xenon, radon, and tritium). Some elements will plate out on cool surfaces. (Tiny quantities of platinum, gold, iridium, etc. can be gained this way.) As for metals that will dissolve in the molten salt, they can be removed with some effort which requires many successive chemical steps. Originally it was intended to remove them constantly, but recently the idea is to let these products build up for a couple years, then remove the salt to a specialized plant to remove these wastes. While the wastes are in the reactor, they will take up a small amount of volume, reducing the reactor's efficiency slightly (trivial), but some are neutron poisons which lower the reactor efficiency (important). (This waste processing is the least developed part of LFTR. It has been designed on paper, but little practical work has been done.)<ref>https://en.wikipedia.org/wiki/Liquid_fluoride_thorium_reactor#Removal_of_fission_products</ref> |
However, if the reactor only burns up (say) 80% of its fuel rather than 100%, the cost of the fuel is still insignificant compared to value you can gain from the reactor. (You can also make the reactor (say) 25% bigger if the amount of energy is a concern.) | However, if the reactor only burns up (say) 80% of its fuel rather than 100%, the cost of the fuel is still insignificant compared to value you can gain from the reactor. (You can also make the reactor (say) 25% bigger if the amount of energy is a concern.) | ||
| Line 64: | Line 68: | ||
==Carbonyl chemical reactions== | ==Carbonyl chemical reactions== | ||
| − | This paper suggests that Th can form carbonyl compounds from carbon monoxide gas.<ref>https://escholarship.org/uc/item/06g0m4mr</ref> Since carbonyl reactors will likely be used on Mars, if thorium can be concentrated this way, it would be a 'free' by-product of pulling nickel, cobalt, iron, titanium, vanadium, ruthenium, chromium, and other metals out of the regolith. (This won't really be free, because each carbonyl would require different temperatures & pressures to form; spending time and power for each. So the reactor would run at one set of conditions to extract one type of metal, and then run at different temperatures and pressures to extract the next. But so long as the thorium carbonyl can be formed within the range of the reactor, it won't require exotic efforts to pull it out of the low grade ore.) | + | This paper suggests that Th can form carbonyl compounds from carbon monoxide gas.<ref>https://escholarship.org/uc/item/06g0m4mr</ref> Since carbonyl reactors will likely be used on Mars, if thorium can be concentrated this way, it would be a 'free' by-product of pulling nickel, cobalt, iron, titanium, vanadium, ruthenium, chromium, and other metals out of the regolith. (This won't really be free, because each carbonyl would require different temperatures & pressures to form; spending time and power for each. So the reactor would run at one set of conditions to extract one type of metal, and then run at different temperatures and pressures to extract the next. But so long as the thorium carbonyl can be formed within the range of the reactor, it won't require exotic efforts to pull it out of the low grade ore.) See [[Metal carbonyl]] for more information. |
==References== | ==References== | ||
Latest revision as of 14:25, 23 September 2024
ref
| Th | ' |
| Thorium | |
Abundance:
Thorium, Periodic table Th, is present on Mars, however, its surface concentration seems to be lower than on Earth.[1] Thorium can be used to produce fuel for nuclear reactors on Mars, including Liquid Fluoride Thorium Reactors, nuclear thermal propulsion and nuclear pulsed propulsion.
Note that Thorium has a very long half life of 14 billion years, so the majority of the thorium that existed when the Earth and Mars were formed is still here (the planets are is 4.5 billion years old). Thorium is not fissile, but it is fertile. In other words, thorium does not spontaneously fission, but with neutron bombardment, it will transform into fissionable U233.
Contents
Concentration of thorium
The average surface concentration is 0,6 ppm, or about ten times lower than Earth' average abundance of 6 ppm, with some high concentration areas of about 1 ppm [2]. See map. Martian basalts may have concentrations of 5 ppm(), similar to the basalts of Earth. Monazite (a phosphate mineral that also includes rare Earth elements) mines on Earth can have a concentration from 500ppm to 25,000 ppm of Thorium. Naturally concentrated deposits are welcome, but are not needed to make Thorium economical on Mars.[3] [4] Mines producing other metals could be utilized, many which produce a waste stream enriched in thorium.[5][6]
This page: Radioactive Rarity on Mars discusses the apparent rarity of radioactive elements on Mars.
Citation Needed
How to breed Th232 into U233
When Th232 is in a reactor core it undergoes neutron bombardment. It can be bred into U233 (an ideal fission fuel better than U235) in two ways:
1). The usual way is for Th232 to absorb a neutron becoming thorium 233. This immediately beta decays to protactinium 233 which has a half life of 27 days. Pa233 beta decays into U233.
2). However, rarely a fast neutron can hit Th232 and 'knock' 2 neutrons free from the thorium, becoming Th231. (This is the n --> 2n decay found in reactor cores.) This beta decays into Pa232 which can absorb a neutron to reach Pa233, and from there beta decay into U233.
If you don't understand the above paragraph, the following page on Isotopes will help.
Note that as the U233 is bred, a small amount of U232 is also created. This decays releasing powerful gamma rays twice in its early decay chain, which is bad and good. (Bad in that the reactor core is even MORE radioactive, good in that in makes U233 less attractive for atom bomb making.)
A molten salt reactor called a LFTR is designed to breed Thorium into U233.
Cost to refine & use thorium
Cost of refining metals
Thorium is about as rare on Earth as lead, and lead is extracted at $2/kg. However, thorium is a nuisance by-product of Rare-Earth Elements (REE) mining, with no commercial use, so it is currently very cheap. (Lead is extracted from sulphides which are cheaper to refine than thorium which is usually found in oxides, so thorium would likely be from 4 to 6 times more expensive to reduce from its ores.) Lead is about 10 parts per million in the Earth's crust. Thorium is about 6 ppm.
However, thorium ores have much higher concentrations: thorianite (rare) is 12% ThO (plus a good deal of useful uranium); monazite is 2.5% Th (25,000 ppm); allanite is 0.1 to 2% Th (1,000 to 20,000 ppm); zircon can have up to 0.4% Th (4,000 ppm).[7]
If we assume that thorium extraction is 6 times the cost of extracting lead, times 1/60% (since Th is slightly rarer than lead) we get a cost of $20 / kg. (Note: Google says that you can buy thorium at $30/kg. Perhaps if thorium was mined in as large quantities as lead, this price would drop. I'll use the google value from this point forward.)
Value of thorium's energy
The specific energy of thorium is 79,420,000 megaJoules / kg.[8] (This is the equivalent of 12,977 barrels of oil - a huge amount of energy.) In the USA an average home uses 10,600 kWh per year. [9]. 1 kiloWatt hour = 3.6e^6 Joules, so 10,600 kWh = 38,160 megaJoules. Dividing this into the specific energy of 1 kg of Thorium, 1 kg of thorium (if completely fissioned, and converted to electrical power at 50% efficiency) would power 1,040 homes for a year. Rounding off, call it a town of 1,000 houses.
As of 2022, the average cost of electricity in the USA is $0.142 per kWh. Multiplying this by 10,600 kWh / home * 1,000 homes = $1,505,000. Using the Google price of thorium at $30 / kg, it can be seen that the fuel price is insignificant compared to the value of the power. (If you want to round off a little, some easy to remember values are: 1 kg of Th will produce ~1 MW (electric) in a year, which is enough to power 1,000 homes, which is worth $1.5 M.)
Thorium on Mars might be 6 to 10 times rarer. If Thorium cost $300 / kg, this $300 is dwarfed by the value of the power generated.
Discussion on the assumptions made in this calculation
We have made 3 major assumptions:
- The assumption that electrical power can be made at 50% efficiency is reasonable. Typical nuclear power plants (operating at lower temperatures) are 32 to 36% efficient. LFTR operate at 700 degrees C and so should get 45% on Earth. But Mars is much colder, so heat engines (which are more efficient if the cold sink is much colder than the hot side of the engine) become more efficient. However, if the reactor gets (say) 40% efficiency, then it does not change things much; the fuel cost remains insignificant.
Also note that on cold Mars, the 'wasted' 50% heat can be used for many jobs. Heating the colony, or being used for industrial process heat are the obvious ones.
- The next assumption that the thorium would burn up 100% is more suspect. Typical fission power plants burn only 0.5% of their fuel before it is 'spent' and sent to long term waste. However, current light water reactors were designed 70 years ago, and are WILDLY inefficient. They use SOLID fuel oxides, clad in zirconium alloy cladding. As the U235 fissions, the fuel elements swell and crack, releasing fission products (especially Xenon gas which is the worse neutron poison). The fuel cylinders swell which puts pressure on the Zr cladding. The zirconium cladding becomes brittle from neutron bombardment. So before they break, the fuel rod is removed (say after 17 months), and a new one is put in its place. The old fuel rods should be dissolved in acid, the fission products discarded, the 99.5% unburnt uranium recovered, converted back into a solid oxide and put back in a new fuel rod. But the fission products are wildly, dangerously radioactive, and this chemical processing is expensive (especially considering the radioactivity). Fresh fuel is so cheap, that it is more profitable to discard the used fuel rods, even tho more than 99% of the uranium has not fissioned.
A LFTR burning thorium has a different philosophy. The unburnt fuel STAYS in the reactor until it 'burns'. The best place for these heavy nucleotides, is inside the reactor. Ideally, the only thing leaving the reactor is heat and fission products. The molten salt is a LIQUID so you can do chemical processes on it directly. Helium gas can be bubbled thru the working salt to pull out gases (such as xenon, radon, and tritium). Some elements will plate out on cool surfaces. (Tiny quantities of platinum, gold, iridium, etc. can be gained this way.) As for metals that will dissolve in the molten salt, they can be removed with some effort which requires many successive chemical steps. Originally it was intended to remove them constantly, but recently the idea is to let these products build up for a couple years, then remove the salt to a specialized plant to remove these wastes. While the wastes are in the reactor, they will take up a small amount of volume, reducing the reactor's efficiency slightly (trivial), but some are neutron poisons which lower the reactor efficiency (important). (This waste processing is the least developed part of LFTR. It has been designed on paper, but little practical work has been done.)[10]
However, if the reactor only burns up (say) 80% of its fuel rather than 100%, the cost of the fuel is still insignificant compared to value you can gain from the reactor. (You can also make the reactor (say) 25% bigger if the amount of energy is a concern.)
- The assumption that power would be as cheap on Mars as on Earth is suspect. If the cost of power on Mars is 10 times higher than on Earth, then the worth of the reactor is multiplied ten fold. (To be fair, the value of solar cells plus batteries, or geothermal power are also multiplied 10 fold.)
If thorium really is 10 times rarer on Mars than on Earth, and we assume that any ores found on Mars are 8 times poorer than that ten fold reduction (for mysterious reasons), then it would cost 80 times more to refine Th on Mars than on Earth ($30/kg). It thus would cost $2,400 / kg to produce thorium on Mars. (Let's round this up to $2,500 / kg.) Given that the reactor produces ~$1,500,000 dollars worth of electricity, the fuel cost remains trivial. Even extremely low quality ores would be able to be burnt at a profit.
Conclusion
Early reactors on Mars, are likely to be much smaller, less efficient, and have less burn thru. Rather than prospecting for radioactives, fuel would be shipped to Mars. But even is thorium really is 6 or 10 times rarer on Mars than on Earth, this won't make nuclear power impossible.
Carbonyl chemical reactions
This paper suggests that Th can form carbonyl compounds from carbon monoxide gas.[11] Since carbonyl reactors will likely be used on Mars, if thorium can be concentrated this way, it would be a 'free' by-product of pulling nickel, cobalt, iron, titanium, vanadium, ruthenium, chromium, and other metals out of the regolith. (This won't really be free, because each carbonyl would require different temperatures & pressures to form; spending time and power for each. So the reactor would run at one set of conditions to extract one type of metal, and then run at different temperatures and pressures to extract the next. But so long as the thorium carbonyl can be formed within the range of the reactor, it won't require exotic efforts to pull it out of the low grade ore.) See Metal carbonyl for more information.
References
"Thorium: Energy Cheaper than Coal", by Robert Hargraves, ISBN 9-781478-161295.
"Molten Salt Reactors and Thorium Energy", Edited by Thomas J. Dolan, ISBN 978-0-08-101126-3.
- ↑ Map of Martian Thorium at Mid-Latitudes, JPL Map of Martian Thorium at Mid-Latitudes , https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.
- ↑ https://en.wikipedia.org/wiki/Occurrence_of_thorium
- ↑ https://aip.scitation.org/doi/pdf/10.1063/1.4972913
- ↑ https://www.youtube.com/watch?v=lxwF93wnRQo
- ↑ https://trace.tennessee.edu/cgi/viewcontent.cgi?article=3397&context=utk_chanhonoproj
- ↑ https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes
- ↑ https://en.wikipedia.org/wiki/Occurrence_of_thorium
- ↑ https://en.wikipedia.org/wiki/Energy_density
- ↑ https://www.eia.gov/tools/faqs/faq.php?id=97&t=3
- ↑ https://en.wikipedia.org/wiki/Liquid_fluoride_thorium_reactor#Removal_of_fission_products
- ↑ https://escholarship.org/uc/item/06g0m4mr
Bazilevskii, A. T., L. P. Moskaleva, O. S. Manvelian, and Iu A. Surkov. "Evaluation of the thorium and uranium contents of Martian surface rock-A new interpretation of Mars-5 gamma-spectroscopy measurements." Geokhimiia (1981): 10-16.