Thorium
ref
| Th | ' |
| Thorium | |
Abundance:
Thorium, Periodic table Th, is present on Mars, however, its surface concentration seems to be lower than on Earth.[1] Thorium can be used to produce fuel for nuclear reactors on Mars, nuclear thermal propulsion and nuclear pulsed propulsion.
Note that Thorium has a very long half life of 14 billion years, so the majority of the thorium that existed when the Earth formed is still here (Earth is 4.5 billion years old). Thorium is not Fissile, but it is Fertile. In other words, thorium does not spontaneously fission, but with neutron bombardment, it will transform into fissionable U233.
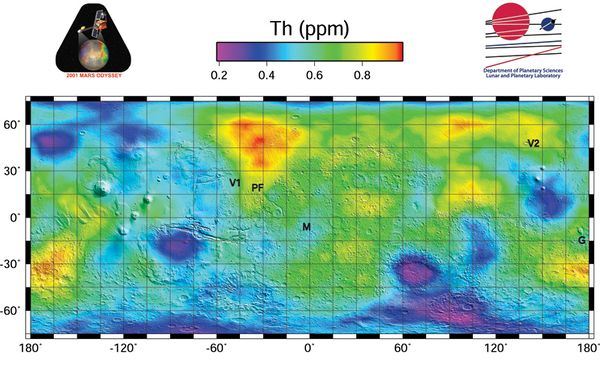
Concentration of thorium
The average surface concentration is 0,6 ppm, or about ten times lower than Earth' average abundance of 6 ppm, with some high concentration areas of about 1 ppm [2]. See map. Martian basalts may have concentrations of 5 ppm(), similar to the basalts of Earth. Monazite (a phosphate mineral that also includes rare Earth elements) mines on Earth can have a concentration of 500ppm of Thorium. Naturally concentrated deposits would need to be found to make the use of Thorium economical on Mars in the long term, or the tailings of rare earth element[3] or other[4] mines could be utilized, which typically produce a waste stream enriched in thorium.
This page: Radioactive Rarity on Mars discusses the apparent rarity of radioactive elements on Mars.
How to breed Th232 into U233
When Th232 is in a reactor core it undergoes neutron bombardment. It can be bred into U233 (an ideal fission fuel better than U235) in two ways:
1). The usual way is for Th232 to absorb a neutron becoming thorium 233. This immediately beta decays to protactinium 233 which has a half life of 40 days. Pa233 beta decays into U233.
2). However, rarely a fast neutron can hit Th232 and 'knock' 2 neutrons free from the thorium, becoming Th231. (This is the n --> 2n 'decay' found in reactor cores.) This beta decays into Pa232 which can absorb a neutron to reach Pa233, and from there beta decay into U233.
Cost to refine
Thorium is about as rare on Earth as lead, and lead is extracted at $2/kg. However, thorium is a nuisance by product of Rare-Earth Elements (REE) mining, with no commercial use, so it is currently very cheap. (Lead is extracted from sulphides which are cheaper to refine than thorium which is usually found in oxides, so thorium would likely be from 2 to 5 times more expensive to reduce from its ores.)
References
- ↑ Map of Martian Thorium at Mid-Latitudes, JPL Map of Martian Thorium at Mid-Latitudes , https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.
- ↑ https://en.wikipedia.org/wiki/Occurrence_of_thorium
- ↑ https://www.youtube.com/watch?v=lxwF93wnRQo
- ↑ https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes
Bazilevskii, A. T., L. P. Moskaleva, O. S. Manvelian, and Iu A. Surkov. "Evaluation of the thorium and uranium contents of Martian surface rock-A new interpretation of Mars-5 gamma-spectroscopy measurements." Geokhimiia (1981): 10-16.