Thorium
ref
| Th | ' |
| Thorium | |
Abundance:
Thorium, Periodic table Th, is present on Mars, however, its surface concentration seems to be lower than on Earth.[1] Thorium can be used to produce fuel for nuclear reactors on Mars, including Liquid Fluoride Thorium Reactors, nuclear thermal propulsion and nuclear pulsed propulsion.
Note that Thorium has a very long half life of 14 billion years, so the majority of the thorium that existed when the Earth formed is still here (Earth is 4.5 billion years old). Thorium is not Fissile, but it is Fertile. In other words, thorium does not spontaneously fission, but with neutron bombardment, it will transform into fissionable U233.
Contents
Concentration of thorium
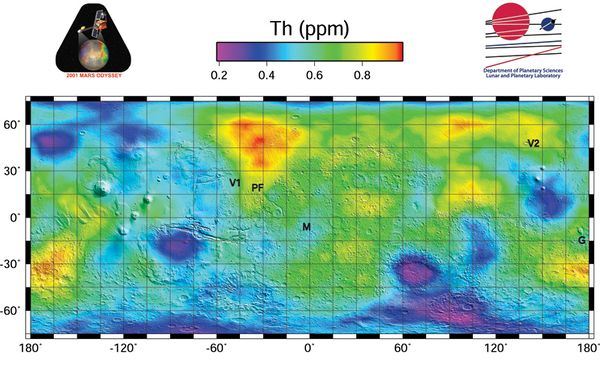
The average surface concentration is 0,6 ppm, or about ten times lower than Earth' average abundance of 6 ppm, with some high concentration areas of about 1 ppm [2]. See map. Martian basalts may have concentrations of 5 ppm(), similar to the basalts of Earth. Monazite (a phosphate mineral that also includes rare Earth elements) mines on Earth can have a concentration of 500ppm of Thorium. Naturally concentrated deposits would need to be found to make the use of Thorium economical on Mars in the long term.<citation needed> Alternately the tailings of rare earth elements mines[3] or other[4] mines could be utilized, which typically produce a waste stream enriched in thorium.
This page: Radioactive Rarity on Mars discusses the apparent rarity of radioactive elements on Mars.
How to breed Th232 into U233
When Th232 is in a reactor core it undergoes neutron bombardment. It can be bred into U233 (an ideal fission fuel better than U235) in two ways:
1). The usual way is for Th232 to absorb a neutron becoming thorium 233. This immediately beta decays to protactinium 233 which has a half life of 27 days. Pa233 beta decays into U233.
2). However, rarely a fast neutron can hit Th232 and 'knock' 2 neutrons free from the thorium, becoming Th231. (This is the n --> 2n decay found in reactor cores.) This beta decays into Pa232 which can absorb a neutron to reach Pa233, and from there beta decay into U233.
Cost to refine
Thorium is about as rare on Earth as lead, and lead is extracted at $2/kg. However, thorium is a nuisance by-product of Rare-Earth Elements (REE) mining, with no commercial use, so it is currently very cheap. (Lead is extracted from sulphides which are cheaper to refine than thorium which is usually found in oxides, so thorium would likely be from 3 to 5 times more expensive to reduce from its ores.) Lead is about 10 parts per million in the Earth's crust. Thorium is about 6 ppm.
However, thorium ores are much higher: thorianite (rare) is 12% ThO (plus a good deal of useful uranium); monazite is 2.5% Th; allanite is 0.1 to 2% Th; zircon can have up to 0.4% Th.[5]
1 kg of Th will produce ~1 MW for a year. As of 2022, the average cost of electricity in the USA is $0.142 per kWh. So that 1 kg of thorium, can produce $1,245 per year. If we assume that thorium extraction is 5 times the cost of extracting lead, times 1/60% (since Th is slightly rarer than lead) we get a cost of ($16.7 / kg), then the fuel cost of thorium is trivial (<1.5%) compared to the value of the power it generates. Thus, if there are thorium ores on Mars, it is certain that a large energy profit can be made from them.
If thorium really is 10 times rarer on Mars than on Earth, and we assume that any ores found on Mars are 3.33 times poorer than that 1/10 (for mysterious reasons), then it would cost 33.3 times more to refine Th on Mars than lead on Earth. In this case, there would STILL be a large energy profit to be made. ($694 profit per kg of thorium assuming energy costs $0.142 / kWh.) Therefore, even extremely low quality ores would be able to be 'burnt' at a profit.
Using these assumptions, if the best thorium ore on Mars is 75 times poorer than those on Earth, there is no energy profit to be made.
Carbonyl reactors
This paper suggests that Th can form carbonyl compounds from carbon monoxide gas.[6] Since carbonyl reactors will likely be used on Mars, if thorium can be concentrated this way, it would be a 'free' by-product of pulling nickel, cobalt, iron, titanium, vanadium, ruthenium, chromium, and other metals out of the regolith. (This won't really be free, because each carbonyl would require different temperatures & pressures to form; spending time and power for each. So the reactor would run at one set of conditions to extract one type of metal, and then run at different temperatures and pressures to extract the next. But so long as the thorium carbonyl can be formed within the range of the reactor, it won't require exotic efforts to pull it out of the low grade ore.)
References
"Thorium: Energy Cheaper than Coal", by Robert Hargraves, ISBN 9-781478-161295.
"Molten Salt Reactors and Thorium Energy", Edited by Thomas J. Dolan, ISBN 978-0-08-101126-3.
- ↑ Map of Martian Thorium at Mid-Latitudes, JPL Map of Martian Thorium at Mid-Latitudes , https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.
- ↑ https://en.wikipedia.org/wiki/Occurrence_of_thorium
- ↑ https://www.youtube.com/watch?v=lxwF93wnRQo
- ↑ https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes
- ↑ https://en.wikipedia.org/wiki/Occurrence_of_thorium
- ↑ https://escholarship.org/uc/item/06g0m4mr
Bazilevskii, A. T., L. P. Moskaleva, O. S. Manvelian, and Iu A. Surkov. "Evaluation of the thorium and uranium contents of Martian surface rock-A new interpretation of Mars-5 gamma-spectroscopy measurements." Geokhimiia (1981): 10-16.