Difference between revisions of "Phoenix Mars Mission"
(→Accomplishments: added ref) |
|||
| Line 40: | Line 40: | ||
==Accomplishments== | ==Accomplishments== | ||
| − | Phoenix has confirmed the presence of water ice in the Martian soil.<ref>Smith, P., et al. 2009. H<sub><sup>2</sub></sup>O at the Phoenix Landing Site. Science: 325, 58-61.</ref> The soil contains small amounts of salt (perchlorate salt, calcium carbonate).<ref>Hecht, M., et al. 2009. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science: 325, 64-67.</ref> <ref>https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20081110.html</ref> <ref>Boynton, W., et al. 2009. Evidence for Calcium Carbonate at the Mars Phoenix Landing Site. Science: 325, 61-64.</ref> <ref>http://science.sciencemag.org/content/325/5936/61?ijkey=tmmqZ1fV6F9z.&keytype=ref&siteid=sci</ref> The mildly alkaline soil environment provides good conditions for growing plants. | + | Phoenix has confirmed the presence of water ice in the Martian soil.<ref>Smith, P., et al. 2009. H<sub><sup>2</sub></sup>O at the Phoenix Landing Site. Science: 325, 58-61.</ref> <ref>https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20080530.html</ref> The soil contains small amounts of salt (perchlorate salt, calcium carbonate).<ref>Hecht, M., et al. 2009. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science: 325, 64-67.</ref> <ref>https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20081110.html</ref> <ref>Boynton, W., et al. 2009. Evidence for Calcium Carbonate at the Mars Phoenix Landing Site. Science: 325, 61-64.</ref> <ref>http://science.sciencemag.org/content/325/5936/61?ijkey=tmmqZ1fV6F9z.&keytype=ref&siteid=sci</ref> The mildly alkaline soil environment provides good conditions for growing plants. |
Perchlorate (ClO<sub>4</sub>) is a strong oxidizing agent; hence, it may be used for rocket fuel and as a source of oxygen.<ref>http://blogs.discovermagazine.com/crux/2016/06/20/perchlorate-salt-mars-surface/#.W-Iv85NKjIV</ref> <ref>https://www.chegg.com/homework-help/ammonium-perchlorate-rocket-fuel-ammonium-perchlorate-solid-chapter-9-problem-1ce-solution-9781133715078-exc</ref> <ref>https://www.quora.com/Could-perchlorate-compounds-on-Mars-be-used-for-rocket-propellant</ref> | Perchlorate (ClO<sub>4</sub>) is a strong oxidizing agent; hence, it may be used for rocket fuel and as a source of oxygen.<ref>http://blogs.discovermagazine.com/crux/2016/06/20/perchlorate-salt-mars-surface/#.W-Iv85NKjIV</ref> <ref>https://www.chegg.com/homework-help/ammonium-perchlorate-rocket-fuel-ammonium-perchlorate-solid-chapter-9-problem-1ce-solution-9781133715078-exc</ref> <ref>https://www.quora.com/Could-perchlorate-compounds-on-Mars-be-used-for-rocket-propellant</ref> | ||
Revision as of 07:57, 28 December 2018
NASA's Phoenix mission was a robotic mission, the first lander in NASA's "Scout class". The lander landed in Mars' north polar region on May 25th 2008, and the scientific package aimed to answer the questions:
- Can the Martian arctic support life?
- What is the history of water at the landing site?
- How is the Martian climate affected by polar dynamics?
The mission name derives from several components built previously for cancelled missions, including 2001's Mars Surveyor lander.
The mission was of considerable interest to planners of human missions to Mars, as many practical mission designs assume the availability of easily-extractable water for fuel production and industrial processes. The polar regions were expected to be the most likely places to find water ice.
Contents
Mission Profile
Phoenix was launched on 4th August 2007 on a Delta II 7925 rocket, and the scheduled landing on Mars on 25th May 2008 was successful. The landing site was the ice-capped northern polar region in the Green Valley of Vastitas Borealis.[1]
Although the primary mission was expected to last around 90 sols, the lander was working 152 sols. The last transmission was received on November 2nd, 2008 by the project team.
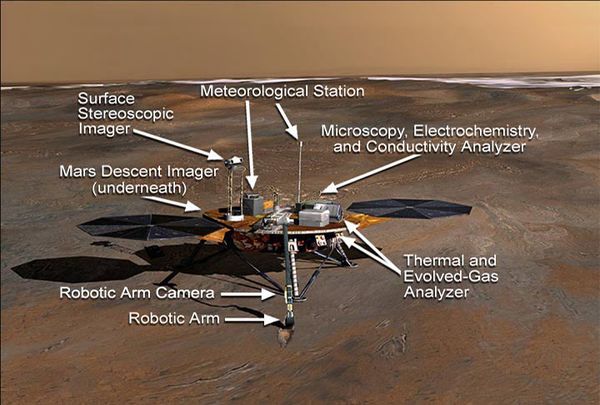
Thermal and Evolved Gas Analyzer
Phoenix's Thermal and Evolved Gas Analyzer (TEGA) is a combination of a furnace and a mass spectrometer. Samples of dirt are heated and the gases given off are analyzed with the mass spectrometer. TEGA measured how much water vapor and carbon dioxide gas were given off. Volatile organic compounds were also able to be detected.[2]
Wet Chemistry Laboratory (WCL)
The Wet Chemistry Laboratory added water to samples that were delivered by the robotic arm. The samples were then stirred. Using a number of electrochemical sensors, the instrument measured ions that were dissolved from the sample.[3]
Accomplishments
Phoenix has confirmed the presence of water ice in the Martian soil.[4] [5] The soil contains small amounts of salt (perchlorate salt, calcium carbonate).[6] [7] [8] [9] The mildly alkaline soil environment provides good conditions for growing plants.
Perchlorate (ClO4) is a strong oxidizing agent; hence, it may be used for rocket fuel and as a source of oxygen.[10] [11] [12]
Analysis of the first soil sample discovered bound water and CO2 These were released during the highest-temperature, 1,000 °C) heating cycle.[13]
Chemicals measured in the samples were chloride, bicarbonate, magnesium, sodium, potassium, calcium, and sulfate. Further data analysis indicated that the soil contains soluble sulfate (SO3) at a minimum of 1.1% and provided a refined formulation of the soil.[14]
Phoenix studied the atmosphere and found snowfall. The laser instrument found snow falling from clouds at about 2.5 miles (4 km). Snow turned to vapor before landing on the ground.[15] Usually, Phoenix measured wind speeds between 5-10 m/s. It was concluded that heat from the sun sublimates ice and adsorbed water from soil, and when temperatures cool, water is returned as snow and frost to the soil. Temperatures went from -30 C to -90 C,[16] [17]
See Also
The Phoenix Mars Mission (MarsHome.org)
References:
- ↑ https://www.webcitation.org/5W4NeGhno?url=http://phoenix.lpl.arizona.edu/
- ↑ Boynton, William V; Bailey, Samuel H; Hamara, David K; Williams, Michael S; Bode, Rolfe C; Fitzgibbon, Michael R; Ko, Wenjeng; Ward, Michael G; Sridhar, K. R; Blanchard, Jeff A; Lorenz, Ralph D; May, Randy D; Paige, David A; Pathare, Asmin V; Kring, David A; Leshin, Laurie A; Ming, Douglas W; Zent, Aaron P; Golden, D. C; Kerry, Kristopher E; Lauer, H. Vern; Quinn, Richard C (2001). "Thermal and Evolved Gas Analyzer: Part of the Mars Volatile and Climate Surveyor integrated payload". Journal of Geophysical Research: Planets. 106 (E8): 17683–98.
- ↑ Kounaves, Samuel P; Lukow, Stefan R; Comeau, Brian P; Hecht, Michael H; Grannan-Feldman, Sabrina M; Manatt, Ken; West, Steven J; Wen, Xiaowen; Frant, Martin; Gillette, Tim (2003). "Mars Surveyor Program '01 Mars Environmental Compatibility Assessment wet chemistry lab: A sensor array for chemical analysis of the Martian soil". Journal of Geophysical Research. 108 (E7): 5077.
- ↑ Smith, P., et al. 2009. H2O at the Phoenix Landing Site. Science: 325, 58-61.
- ↑ https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20080530.html
- ↑ Hecht, M., et al. 2009. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science: 325, 64-67.
- ↑ https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20081110.html
- ↑ Boynton, W., et al. 2009. Evidence for Calcium Carbonate at the Mars Phoenix Landing Site. Science: 325, 61-64.
- ↑ http://science.sciencemag.org/content/325/5936/61?ijkey=tmmqZ1fV6F9z.&keytype=ref&siteid=sci
- ↑ http://blogs.discovermagazine.com/crux/2016/06/20/perchlorate-salt-mars-surface/#.W-Iv85NKjIV
- ↑ https://www.chegg.com/homework-help/ammonium-perchlorate-rocket-fuel-ammonium-perchlorate-solid-chapter-9-problem-1ce-solution-9781133715078-exc
- ↑ https://www.quora.com/Could-perchlorate-compounds-on-Mars-be-used-for-rocket-propellant
- ↑ http://www.planetary.org/blogs/emily-lakdawalla/2008/1526.html
- ↑ Kounaves, Samuel P; Hecht, Michael H; Kapit, Jason; Quinn, Richard C; Catling, David C; Clark, Benton C; Ming, Douglas W; Gospodinova, Kalina; Hredzak, Patricia; McElhoney, Kyle; Shusterman, Jennifer (2010). "Soluble sulfate in the martian soil at the Phoenix landing site". Geophysical Research Letters. 37 (9): L09201.
- ↑ https://www.nasa.gov/mission_pages/phoenix/news/phoenix-20080929.html
- ↑ http://adsabs.harvard.edu/abs/2010cosp...38.1365S
- ↑ Smith, P. 2010. Atmospheric results from the Phoenix Mars Mission. 38th COSPAR Scientific Assembly. Held 18-15 July 2010, in Bremen, Germany, p.2.