Difference between revisions of "Air"
(→Oxygen) |
|||
| Line 10: | Line 10: | ||
===[[Oxygen]]=== | ===[[Oxygen]]=== | ||
| − | [[Oxygen]] is the one essential component of any breathing gas. At sea level on Earth, the partial pressure of oxygen is about 22 kPa | + | [[Oxygen]] is the one essential component of any breathing gas. At sea level on Earth, the partial pressure of oxygen is about 22 kPa. Habitats on Mars will likely have a similar concentration. High oxygen concentrations and high oxygen partial pressures are possible. However, both contribute to increased flammability, so the choice of reducing pressure by increasing oxygen partial pressure has important consequences. |
===Inert gases=== | ===Inert gases=== | ||
| − | [[Nitrogen]] and [[argon]] are available in similar concentrations in Mars’ atmosphere and would both be suitable for use in habitats. Because inert gases slow the spread fire by absorbing heat, and nitrogen has about 65% more heat capacity per volume than argon<ref>[http://hyperphysics.phy-astr.gsu.edu/hbase/Tables/heatcap.html Molar Heat Capacities, Gases]</ref>, nitrogen may be preferred. But it is also plausible that a nitrogen/argon mix would be used since the mix would be easier to obtain, or that argon would be used, since nitrogen has other uses, such as the production of fertilizer. | + | [[Nitrogen]] and [[argon]] are available in similar concentrations in Mars’ atmosphere and would both be suitable for use in habitats. Because inert gases slow the spread fire by absorbing heat, and nitrogen has about 65% more heat capacity per volume than argon<ref>[http://hyperphysics.phy-astr.gsu.edu/hbase/Tables/heatcap.html Molar Heat Capacities, Gases]</ref>, nitrogen may be preferred. But it is also plausible that a nitrogen/argon mix would be used since the mix would be easier to obtain, or that argon would be used, since nitrogen has other uses, such as the production of fertilizer. As argon is denser than the other gases in an atmospheric mix, a risk exists that rooms with pour air circulation might accumulate excess argon, that by displacing oxygen might increase the risk of suffocation. |
===[[Carbon dioxide]]=== | ===[[Carbon dioxide]]=== | ||
| − | Carbon dioxide (CO<sub>2</sub>) is a low concentration atmospheric component in the habitat, produced by the human metabolism and industrial processes. Excess carbon dioxide concentrations can produce a variety of negative health effects, even at low concentrations. But CO<sub>2</sub> is a also a requirement for plant metabolism. CO<sub>2</sub> level on | + | Carbon dioxide (CO<sub>2</sub>) is a low concentration atmospheric component in the habitat, produced by the human metabolism, plants and industrial processes. Excess carbon dioxide concentrations can produce a variety of negative health effects, even at low concentrations. But CO<sub>2</sub> is a also a requirement for plant metabolism. CO<sub>2</sub> level on Earth are increasing gradually, and have reached about 400 ppm, starting from an historical value of about 300 ppm. CO<sub>2</sub> concentration in buildings is often used for fresh air control, with the upper limit set at 1000 ppm. A study on astronauts on the International Space Station found that headache risk was significantly affected by CO<sub>2</sub> levels even at concentrations below 10,000 ppm<ref>[https://journals.lww.com/joem/Abstract/2014/05000/Relationship_Between_Carbon_Dioxide_Levels_and.4.aspx Relationship Between Carbon Dioxide Levels and Reported Headaches on the International Space Station]</ref>. Nuclear submarines can operate with up to 9000 ppm in their atmosphere. In Mars habitats, carbon dioxide will have to be separated and removed from the air, or converted back into oxygen by plants. |
===Water vapor=== | ===Water vapor=== | ||
| − | Water vapor is a product of evaporation, respiration, and combustion processes. At normal atmospheric pressure and temperature, most water condenses out of the atmosphere. However, depending on temperature and humidity, water can represent from 0 to 3% of the atmospheric mass. | + | Water vapor is a product of evaporation, respiration, and combustion processes. At normal atmospheric pressure and temperature, most water condenses out of the atmosphere. However, depending on temperature and humidity, water can represent from 0 to 3% of the atmospheric mass. Water vapor is essential for comfort, and is generally presented as a value of relative humidity. Plants and people produce large amounts of water vapor, that needs to be removed to avoid excessive humidity in the settlement. Water in the atmosphere can condense out on cold surfaces, which may create maintenance problems. |
==Pressure== | ==Pressure== | ||
| Line 63: | Line 63: | ||
Air convection is one of the main heat transfer mechanisms. Reduced pressure air has less capacity for convective heat transfer, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat and evaporation from their surface. A reduction of 50% in atmospheric density corresponds to a reduction in heat capacity of the air of 50%. | Air convection is one of the main heat transfer mechanisms. Reduced pressure air has less capacity for convective heat transfer, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat and evaporation from their surface. A reduction of 50% in atmospheric density corresponds to a reduction in heat capacity of the air of 50%. | ||
| − | === Process impacts === | + | ===Process impacts=== |
A number of processes depend on atmospheric pressure to function correctly. For example, the available suctions head for pumps is a factor of atmospheric pressure. Self priming pumps will have less available head, and may require modifications or have a limited operational range. Processes requiring capillarity may also be affected by reduced pressure. Vacuum system will be less efficient as well and transport dust less efficiently. The ability of the air to absorb moisture will also be lowered by a reduced air pressure and this might affect plant growth or various processes requiring drying. | A number of processes depend on atmospheric pressure to function correctly. For example, the available suctions head for pumps is a factor of atmospheric pressure. Self priming pumps will have less available head, and may require modifications or have a limited operational range. Processes requiring capillarity may also be affected by reduced pressure. Vacuum system will be less efficient as well and transport dust less efficiently. The ability of the air to absorb moisture will also be lowered by a reduced air pressure and this might affect plant growth or various processes requiring drying. | ||
Revision as of 08:58, 26 January 2021
This article is for air inside a Martian settlement. For the air outside, see atmosphere.
Settlers on Mars will depend on manufactured air for breathing, since the planet's atmosphere is too thin and lacks Oxygen. This air will be provided and controlled by the settlement life support system.
Standard air on Earth is composed of Nitrogen (78%) and Oxygen (21%), with traces of other gases at 101,3 kPa (14,7 psi) of pressure.
Contents
Gases
Oxygen
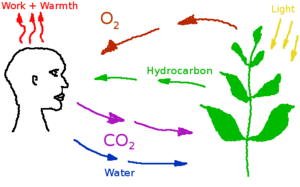
Oxygen is the one essential component of any breathing gas. At sea level on Earth, the partial pressure of oxygen is about 22 kPa. Habitats on Mars will likely have a similar concentration. High oxygen concentrations and high oxygen partial pressures are possible. However, both contribute to increased flammability, so the choice of reducing pressure by increasing oxygen partial pressure has important consequences.
Inert gases
Nitrogen and argon are available in similar concentrations in Mars’ atmosphere and would both be suitable for use in habitats. Because inert gases slow the spread fire by absorbing heat, and nitrogen has about 65% more heat capacity per volume than argon[1], nitrogen may be preferred. But it is also plausible that a nitrogen/argon mix would be used since the mix would be easier to obtain, or that argon would be used, since nitrogen has other uses, such as the production of fertilizer. As argon is denser than the other gases in an atmospheric mix, a risk exists that rooms with pour air circulation might accumulate excess argon, that by displacing oxygen might increase the risk of suffocation.
Carbon dioxide
Carbon dioxide (CO2) is a low concentration atmospheric component in the habitat, produced by the human metabolism, plants and industrial processes. Excess carbon dioxide concentrations can produce a variety of negative health effects, even at low concentrations. But CO2 is a also a requirement for plant metabolism. CO2 level on Earth are increasing gradually, and have reached about 400 ppm, starting from an historical value of about 300 ppm. CO2 concentration in buildings is often used for fresh air control, with the upper limit set at 1000 ppm. A study on astronauts on the International Space Station found that headache risk was significantly affected by CO2 levels even at concentrations below 10,000 ppm[2]. Nuclear submarines can operate with up to 9000 ppm in their atmosphere. In Mars habitats, carbon dioxide will have to be separated and removed from the air, or converted back into oxygen by plants.
Water vapor
Water vapor is a product of evaporation, respiration, and combustion processes. At normal atmospheric pressure and temperature, most water condenses out of the atmosphere. However, depending on temperature and humidity, water can represent from 0 to 3% of the atmospheric mass. Water vapor is essential for comfort, and is generally presented as a value of relative humidity. Plants and people produce large amounts of water vapor, that needs to be removed to avoid excessive humidity in the settlement. Water in the atmosphere can condense out on cold surfaces, which may create maintenance problems.
Pressure
It may be worthwhile to keep Mars habitats at a lower pressure than we generally experience on Earth. This was done on the Apollo and Skylab missions, which both had total pressures of 5 psi (34 kPa). Robert Zubrin advocates for a Skylab-type habitat air mix on Mars, with 3.5 psi (24 kPa) O2 and 1.5 psi (10 kPa) N2[3]. However, the ISS operates at standard atmospheric pressure, as did the Space shuttle. There are several key considerations in determining the optimal air pressure.
Structural stress
Using sea level Earth air pressure, the force on each square meter of a habitat’s surface would be around 100 kN , or 10 tonnes of force per m2. Habitats on Mars will need to have high tensile strength to withstand this great force. Using a lower pressure would reduce the strain, possibly leading to more lightweight and less expensive habitats.
Oxygen partial pressure
The level of oxygen in the air must be high enough to supply sufficient oxygen to the bloodstream. To do this, the partial pressure of oxygen reaching the alveoli in the lungs must be comparable to what we experience on Earth. Because our lungs are saturated with water vapor, oxygen is partially crowded out at very low total pressures, so at those pressures, the partial pressure of oxygen in the air required to properly supply our lungs is actually higher.
| Total pressure (kPa) | Oxygen partial pressure (kPa) | Percent oxygen |
|---|---|---|
| 25.5 | 25.5 | 100 |
| 34.5 | 23.8 | 69.0 |
| 48.3 | 22.7 | 47.0 |
| 62.1 | 22.1 | 35.5 |
| 101.4 | 21.2 | 21.0 |
Since humans can survive at pressures significantly below sea level on Earth, lower oxygen pressures than shown above would certainly be tolerable. However, physical and mental performance are diminished at high altitudes on Earth, so the same is likely true for partial pressures significantly below those in the chart.
Flammability
Flammability is influenced by both the concentration (percentage) and partial pressure of oxygen in an environment, with concentration having the greater effect[5]. So for a given partial pressure of oxygen, reducing the total pressure increases the fire risk.
Heat transfer
Air convection is one of the main heat transfer mechanisms. Reduced pressure air has less capacity for convective heat transfer, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat and evaporation from their surface. A reduction of 50% in atmospheric density corresponds to a reduction in heat capacity of the air of 50%.
Process impacts
A number of processes depend on atmospheric pressure to function correctly. For example, the available suctions head for pumps is a factor of atmospheric pressure. Self priming pumps will have less available head, and may require modifications or have a limited operational range. Processes requiring capillarity may also be affected by reduced pressure. Vacuum system will be less efficient as well and transport dust less efficiently. The ability of the air to absorb moisture will also be lowered by a reduced air pressure and this might affect plant growth or various processes requiring drying.
Open Issue
- What is known about the behaviour of dusty air under low gravity?
References
- ↑ Molar Heat Capacities, Gases
- ↑ Relationship Between Carbon Dioxide Levels and Reported Headaches on the International Space Station
- ↑ Zubrin, Robert (2011). The Case for Mars: The Plan to Settle the Red Planet and Why We Must (2nd ed.) p. 159
- ↑ Guidelines and Capabilities for Designing Human Missions
- ↑ Oxygen Partial Pressure and Oxygen Concentration Flammability: Can They Be Correlated?