Difference between revisions of "Triple point"
(add a physics article on triple point - perhaps we need a chemistry cat?) |
|||
| Line 4: | Line 4: | ||
| − | [[category: | + | [[category:Atmospheric Sciences]] |
Latest revision as of 11:39, 24 July 2018
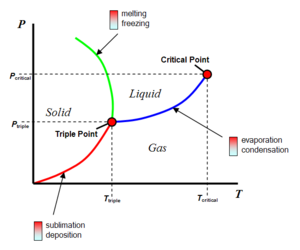
The triple point is the point at which a substance can exist as a solid, liquid and gas at the same time when in thermal equilibrium. In the case of water on the surface of Mars, it can only exist as either a solid or a gas, as the atmospheric pressure is too low to support liquid water. Therefore, Mars atmospheric pressure exists at pressures below the triple point for water. Sublimation occurs at the interface between ice and water vapour as Mars surface ice is heated, there is no liquid phase.