Difference between revisions of "Graphene"
ChristiaanK (talk | contribs) (Created page.) |
ChristiaanK (talk | contribs) |
||
| Line 2: | Line 2: | ||
==Properties== | ==Properties== | ||
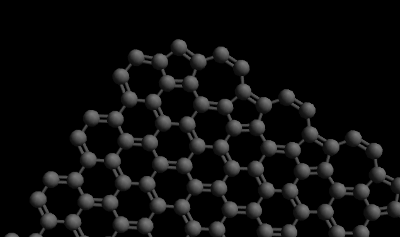
| + | [[File:Graphene_heptagon_pentagon_edge.png|frame|right|The edge on the right of this graphene sheet has been turned into a pattern of heptagons and pentagons by a microscope.]] | ||
Graphene is optically almost transparent<ref name=Housecroft_Sharpe>C.E. Housecroft & A.G. Sharpe - ''Inorganic chemistry'' 2012. ISBN 978-0-273-74275-3. pp. 1056-1058.</ref>.<br /> | Graphene is optically almost transparent<ref name=Housecroft_Sharpe>C.E. Housecroft & A.G. Sharpe - ''Inorganic chemistry'' 2012. ISBN 978-0-273-74275-3. pp. 1056-1058.</ref>.<br /> | ||
Strictly speaking, graphene is a [[semiconductor]], but it is important to note that this does not imply low conductivity. In fact, graphene has the best electrical conductivity at room temperature of any known material<ref name=Fuhrer>[http://www.nature.com/nnano/journal/v3/n4/full/nnano.2008.58.html M.S. Fuhreer ''et al.''] - ''Intrinsic and extrinsic performance limits of graphene devices on SiO2'' 2008. Nature nanotechnology. Vol. 3 no. 4. pp. 206-209. As quoted by [https://newsdesk.umd.edu/scitech/release.cfm?ArticleID=1621 L. Tune] - ''Physicists show electrons can travel more than 100 times faster in graphene'' 2008. Accessed 2013-04-27.</ref>. The current density in graphene is two to three orders of magnitude better current density than copper<ref name=Housecroft_Sharpe />. This low resistivity exists because the unique properties of graphene cause electrons to behave as massless particles, moving at very nearly the [[speed of light]]<ref name=Housecroft_Sharpe /><ref name=Fuhrer />.<br /> | Strictly speaking, graphene is a [[semiconductor]], but it is important to note that this does not imply low conductivity. In fact, graphene has the best electrical conductivity at room temperature of any known material<ref name=Fuhrer>[http://www.nature.com/nnano/journal/v3/n4/full/nnano.2008.58.html M.S. Fuhreer ''et al.''] - ''Intrinsic and extrinsic performance limits of graphene devices on SiO2'' 2008. Nature nanotechnology. Vol. 3 no. 4. pp. 206-209. As quoted by [https://newsdesk.umd.edu/scitech/release.cfm?ArticleID=1621 L. Tune] - ''Physicists show electrons can travel more than 100 times faster in graphene'' 2008. Accessed 2013-04-27.</ref>. The current density in graphene is two to three orders of magnitude better current density than copper<ref name=Housecroft_Sharpe />. This low resistivity exists because the unique properties of graphene cause electrons to behave as massless particles, moving at very nearly the [[speed of light]]<ref name=Housecroft_Sharpe /><ref name=Fuhrer />.<br /> | ||
Revision as of 01:44, 28 April 2013
Graphene is a form of carbon, and may informally be thought of as "perfect" graphite. More specifically, graphene is graphite in which there are no chemical bonds between the individual sheets of graphene, as there normally are in graphite.
Contents
Properties
Graphene is optically almost transparent[1].
Strictly speaking, graphene is a semiconductor, but it is important to note that this does not imply low conductivity. In fact, graphene has the best electrical conductivity at room temperature of any known material[2]. The current density in graphene is two to three orders of magnitude better current density than copper[1]. This low resistivity exists because the unique properties of graphene cause electrons to behave as massless particles, moving at very nearly the speed of light[1][2].
When the edge of a graphene sheet is examined with a transmission electron microscope, the microscope disrupts the edge and creates a boundary of alternating pentagonal and heptagonal structures.[1]
Graphene oxide
One derivative of graphene is graphene oxide, imperfect sheets of graphene bound to oxygen atoms. It can be formed by oxidising graphene with certain acids. Unlike graphene, it is an elecrical insulator[1].
The controlled reduction of graphene oxide can be used to produce materials with electrical properties in between those of graphene and graphene oxide.
Production
Small amounts of graphene can be created from graphite with the use of adhesive tape, silica and a microscope. The adhesive tape sticks to the topmost layer of graphite. and the weak bonding between adjacent layers of graphite will break when the tape is pulled off, taking a layer of graphene with it. The graphene can then be deposited on a substrate. The microscope is needed because multiple layers of graphite come off in a percentage of cases, and these need to be identified and removed.[1]
One of the promising ways to increase graphene production is to grow layers of graphene on a wafer of silicon carbide[1].
Potential use in spaceflight
- With its low density and extreme electrical conductivity, graphene could improve the efficiency of almost all electrical systems on a spacecraft.
- Semiconductors based on graphene and/or graphene oxide may someday result in superior computers.
- Along with carbon nanotubes and carbon fibre, graphene is a good material for spacecraft construction due to its low mass, high strength, low thermal expansion and resistance to thermal shock.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 C.E. Housecroft & A.G. Sharpe - Inorganic chemistry 2012. ISBN 978-0-273-74275-3. pp. 1056-1058.
- ↑ 2.0 2.1 M.S. Fuhreer et al. - Intrinsic and extrinsic performance limits of graphene devices on SiO2 2008. Nature nanotechnology. Vol. 3 no. 4. pp. 206-209. As quoted by L. Tune - Physicists show electrons can travel more than 100 times faster in graphene 2008. Accessed 2013-04-27.