Difference between revisions of "Sublimation"
(→See Also: added to list) |
(Corrected the amount of CO2 deposited each year.) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
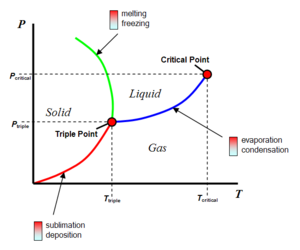
[[Image:Phase_diagram_water.png|thumb|right|300px|The phase diagram for water, clearly displaying water's '''triple point'''. The transition between solid to gas is known as '''sublimation'''.]] | [[Image:Phase_diagram_water.png|thumb|right|300px|The phase diagram for water, clearly displaying water's '''triple point'''. The transition between solid to gas is known as '''sublimation'''.]] | ||
| − | '''Sublimation''' is the phase transition between solid and gas, without an itermediate liquid phase.<ref>Heimler, C., J. Price. 1987. Focus on Physical Science. Merrill. Columbus.</ref> As the pressure and temperature of Mars is very low, water on the surface can only exist as ice or water vapour. In the phase diagram for [[water]] on [[Mars]], temperatures and atmospheric pressure are below the [[triple point]]. | + | '''Sublimation''' is the phase transition between solid and gas, without an itermediate liquid phase.<ref>Heimler, C., J. Price. 1987. Focus on Physical Science. Merrill. Columbus.</ref> As the pressure and temperature of Mars is very low, water on the surface can only exist as ice or water vapour. In the phase diagram for [[water]] on [[Mars]], temperatures and atmospheric pressure are close to the triple point of water. (On warm days it is well below the [[triple point]].) |
Sublimation occurs when ice is heated. A terrestrial example of sublimation is if "dry ice" (solid [[carbon dioxide|CO<sub>2</sub>]]) is exposed to air at one atmosphere. The ice will turn into a gas and appear to steam without passing through a liquid phase. The opposite to sublimation is ''deposition'', where water vapour is cooled and deposited as ice. An example of deposition is terrestrial frost, where water vapour (during winter) becomes very cold, bypassing the liquid (rain) phase. | Sublimation occurs when ice is heated. A terrestrial example of sublimation is if "dry ice" (solid [[carbon dioxide|CO<sub>2</sub>]]) is exposed to air at one atmosphere. The ice will turn into a gas and appear to steam without passing through a liquid phase. The opposite to sublimation is ''deposition'', where water vapour is cooled and deposited as ice. An example of deposition is terrestrial frost, where water vapour (during winter) becomes very cold, bypassing the liquid (rain) phase. | ||
| Line 10: | Line 10: | ||
| − | During the Martian winter CO<sub>2</sub> frost is deposited at the winter pole. During the Martian summer CO<sub>2</sub> ice sublimes from the summer pole. The amount of solid carbon dioxide involved is enormous | + | During the Martian winter CO<sub>2</sub> frost is deposited at the winter pole. During the Martian summer CO<sub>2</sub> ice sublimes from the summer pole. The amount of solid carbon dioxide (CO2) involved is enormous, where 30% of the atmosphere is deposited each Southern winter (and 12% during Northern winter).<ref>Antonio Genova, Sander Goossens, Frank G. Lemoine, Erwan Mazarico, Gregory A. Neumann, David E. Smith, Maria T. Zuber. Seasonal and static gravity field of Mars from MGS, Mars Odyssey and MRO radio science. Icarus, 2016; 272: 228 DOI: 10.1016/j.icarus.2016.02.05</ref> <ref>NASA/Goddard Space Flight Center. "New gravity map gives best view yet inside Mars." ScienceDaily. ScienceDaily, 21 March 2016. </ref> (In the summers, this CO2 sublimes.). This process causes great pressure changes and high winds. |
Sublimation of water ice and dry ice cause many interesting landscape features on Mars. For examples of these forms go to [[Sublimation landscapes on Mars]]. | Sublimation of water ice and dry ice cause many interesting landscape features on Mars. For examples of these forms go to [[Sublimation landscapes on Mars]]. | ||
==See also== | ==See also== | ||
| − | + | ||
*[[Geography of Mars]] | *[[Geography of Mars]] | ||
| − | |||
*[[Glaciers on Mars]] | *[[Glaciers on Mars]] | ||
| + | *[[Martian features that are signs of water ice]] | ||
| + | *[[Sublimation landscapes on Mars]] | ||
==References== | ==References== | ||
Latest revision as of 13:44, 21 May 2021
Sublimation is the phase transition between solid and gas, without an itermediate liquid phase.[1] As the pressure and temperature of Mars is very low, water on the surface can only exist as ice or water vapour. In the phase diagram for water on Mars, temperatures and atmospheric pressure are close to the triple point of water. (On warm days it is well below the triple point.)
Sublimation occurs when ice is heated. A terrestrial example of sublimation is if "dry ice" (solid CO2) is exposed to air at one atmosphere. The ice will turn into a gas and appear to steam without passing through a liquid phase. The opposite to sublimation is deposition, where water vapour is cooled and deposited as ice. An example of deposition is terrestrial frost, where water vapour (during winter) becomes very cold, bypassing the liquid (rain) phase.
Some other examples of ice sublimated are snow slowly disappearing even in low temperatures. In addition, if ice cubes are left in ice trays for a long time in a refrigerator, they can be observed to get smaller and smaller until they disappear. This is an example of sublimation. Naphthalene, an organic compound commonly found in mothballs, sublimes easily because it is made of non-polar molecules that are held together only by weak intermolecular forces (Van der Waals). The element Iodine sublimates easily.[2] Often iodine crystals are found on the top lid of a bottle of iodine. The iodine sublimates from crystals at the bottom of the bottle and then are deposited on the top. So the iodine seems to go right through the air. In a way it’s like the transporter on board the Starship Enterprise—matter is transported from one place to another.
During the Martian winter CO2 frost is deposited at the winter pole. During the Martian summer CO2 ice sublimes from the summer pole. The amount of solid carbon dioxide (CO2) involved is enormous, where 30% of the atmosphere is deposited each Southern winter (and 12% during Northern winter).[3] [4] (In the summers, this CO2 sublimes.). This process causes great pressure changes and high winds.
Sublimation of water ice and dry ice cause many interesting landscape features on Mars. For examples of these forms go to Sublimation landscapes on Mars.
See also
- Geography of Mars
- Glaciers on Mars
- Martian features that are signs of water ice
- Sublimation landscapes on Mars
References
- ↑ Heimler, C., J. Price. 1987. Focus on Physical Science. Merrill. Columbus.
- ↑ Heimler, C., J. Price. 1987. Focus on Physical Science. Merrill. Columbus.
- ↑ Antonio Genova, Sander Goossens, Frank G. Lemoine, Erwan Mazarico, Gregory A. Neumann, David E. Smith, Maria T. Zuber. Seasonal and static gravity field of Mars from MGS, Mars Odyssey and MRO radio science. Icarus, 2016; 272: 228 DOI: 10.1016/j.icarus.2016.02.05
- ↑ NASA/Goddard Space Flight Center. "New gravity map gives best view yet inside Mars." ScienceDaily. ScienceDaily, 21 March 2016.