Difference between revisions of "Air"
m (Just fixing a typo) |
|||
| Line 14: | Line 14: | ||
===Carbon dioxide=== | ===Carbon dioxide=== | ||
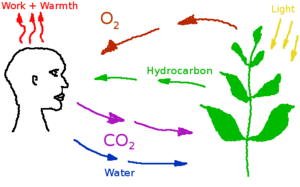
| − | Carbon dioxide is | + | Carbon dioxide is a low concentration atmospheric component produced by the human metabolism and industrial processes. Excess carbon dioxide concentrations can produce a variety of negative health effects, even at low concentrations. But CO<sub>2</sub> is a also a requirement for plant metabolism. A study on astronauts on the International Space Station found that headache risk was significantly affected by CO<sub>2</sub> levels even at concentrations below 1%<ref>https://journals.lww.com/joem/Abstract/2014/05000/Relationship_Between_Carbon_Dioxide_Levels_and.4.aspx</ref> or10 000 (ppm). Nuclear submarines can operate with up to 9000 ppm in their atmosphere. In Mars habitats carbon dioxide will have to be separated and removed, or converted back into oxygen by plants. |
==Low pressure effects== | ==Low pressure effects== | ||
| Line 25: | Line 25: | ||
==Heat transfer== | ==Heat transfer== | ||
| − | Air convection is one of the main heat transfer mechanisms. Reduced density air has less heat carrying capacity, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat | + | Air convection is one of the main heat transfer mechanisms. Reduced density air has less heat carrying capacity, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat and evaporation from their surface. |
==Open Issues== | ==Open Issues== | ||
Revision as of 04:07, 3 June 2019
Settlers on Mars will depend on manufactured air for breathing, since the planet's atmosphere is too thin and lacks Oxygen.
Standard air on Earth is composed of Nitrogen (78%) and Oxygen (21%), with traces of other gases at 101,3 kPa (14,7 psi) of pressure.
Contents
Gases
Oxygen
Oxygen is the one essential component of any breathing gas. At sea level on Earth, the partial pressure of oxygen is about 22 kPa, habitats on Mars will likely have a similar amount. However, high oxygen concentrations and high oxygen partial pressures both contribute to increased flammability, so it may be best to supplement oxygen with other inert gases.
Inert gases
Nitrogen and argon are available in similar concentrations in Mars’ atmosphere and would both be suitable for use in habitats. Because inert gases slow the spread fire by absorbing heat, and nitrogen has about 65% more heat capacity per volume than argon, nitrogen may be preferred. But it is also plausible that a nitrogen/argon mix would be used since the mix would be easier to obtain, or that argon would be used, since nitrogen has other uses like production of fertilizer.
Carbon dioxide
Carbon dioxide is a low concentration atmospheric component produced by the human metabolism and industrial processes. Excess carbon dioxide concentrations can produce a variety of negative health effects, even at low concentrations. But CO2 is a also a requirement for plant metabolism. A study on astronauts on the International Space Station found that headache risk was significantly affected by CO2 levels even at concentrations below 1%[1] or10 000 (ppm). Nuclear submarines can operate with up to 9000 ppm in their atmosphere. In Mars habitats carbon dioxide will have to be separated and removed, or converted back into oxygen by plants.
Low pressure effects
The human breathing works best at terrestrial sea level with an air pressure of 101,3 kilopascal (kPa). The air pressure on the Mount Everest is only 34,0 kPa.
In such high altitudes of the terrestrial atmosphere the air pressure drops to dangerous values, resulting in acute mountain sickness (AMS) and high altitude pulmonary edema (HAPE).
Oxygen reduction for fire prevention
The terrestrial atmosphere contains 21% oxygen, which is the value that human beings have adapted to during a long evolution process. But there is some tolerance. Under normal air pressure persons can live and work with down to 13% oxygen. The danger of fire is much lower in a low oxygen air. With 15% oxygen even paper can no longer burn with a flame. [2]
Heat transfer
Air convection is one of the main heat transfer mechanisms. Reduced density air has less heat carrying capacity, and added ventilation is required for work in low density air. Most plants function more efficiently if there is air movement to remove heat and evaporation from their surface.
Open Issues
- What air pressure, combined with different oxygen levels, is required for persons to survive?
- What air pressure, combined with different oxygen levels, is required for persons to live and work?
- What are the results from the Biosphere 2 experiment? Ideas for mitigation and/or compensation?
- What is known about the behaviour of dusty air under low gravity?