Difference between revisions of "Acetic acid"
(grammar) |
|||
| Line 1: | Line 1: | ||
| + | [[File:Acetic acid 2.svg|thumb|Acetic acid molecule]] | ||
'''Acetic acid''' is a weak [[acid]], and the defining ingredient in vinegar. Aside from its common use in [[food]], acetic acid is an important industrial chemical. | '''Acetic acid''' is a weak [[acid]], and the defining ingredient in vinegar. Aside from its common use in [[food]], acetic acid is an important industrial chemical. | ||
| Line 10: | Line 11: | ||
[[Methanol]] and [[carbon monoxide]] are combined under pressure, in the presence of a [[catalyst]]. | [[Methanol]] and [[carbon monoxide]] are combined under pressure, in the presence of a [[catalyst]]. | ||
=====Ethylene Oxidation===== | =====Ethylene Oxidation===== | ||
| + | [[File:Process-Acetic acid.png|thumb|642x642px|Acetic Acid production process]] | ||
[[Ethylene]] is [[oxidized|oxidized]] in the presence of a [[palladium]] based catalyst. | [[Ethylene]] is [[oxidized|oxidized]] in the presence of a [[palladium]] based catalyst. | ||
=====Ethane Oxidation===== | =====Ethane Oxidation===== | ||
Revision as of 18:45, 25 May 2021
Acetic acid is a weak acid, and the defining ingredient in vinegar. Aside from its common use in food, acetic acid is an important industrial chemical.
Contents
Production
Biological Synthesis
Since prehistoric times, humans have produced acetic acid by exposing alcoholic beverages to aerobic bacteria usually of the acetobacter genus. The bacteria convert the alcohol to acetic acid. Distillation of the vinegar concentrates the acid.
Artificial Synthesis
There are several techniques used to produce acetic acid on an industrial scale.
Methanol Carbonylation
Methanol and carbon monoxide are combined under pressure, in the presence of a catalyst.
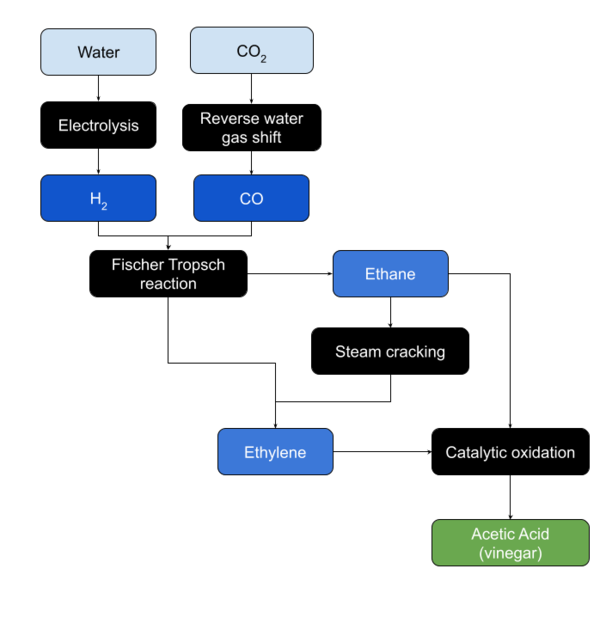
Ethylene Oxidation
Ethylene is oxidized in the presence of a palladium based catalyst.
Ethane Oxidation
Ethane is oxidized in the presence of a catalyst.
Use
Food Preservation
Though acetic acid is commonly generated by certain microbes, in high enough concentrations it preserves food.
Chemical Production
Acetic acid has the formula CH3COOH, and is used in the production of adheisives, inks, paints, acetic anhydride (used in synthetic textiles, photographic film, and flexible plastic type materials), it is an excellent polar protic solvent, and (its largest use on Earth) vinyl acetate monomer.
Medical Use
Since ancient times, it has been used as a medicine to reduce headaches and act is a weak blood thinner. See asprin.