Ethylene
Ethylene, C2H4, is the simplest alkene, a type of hydrocarbon. Ethylene is an important building block used to produce polystyrene, polyethylene, and detergents. Ethylene should be an important product of In-stiu resources utilization.
In situ Production
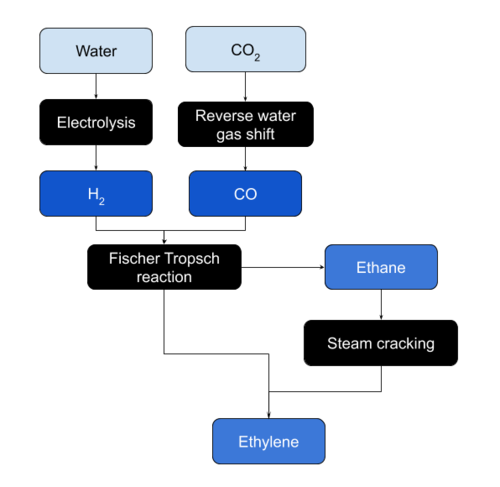
Ethylene can be produced on Mars from Ethane by steam cracking or directly by the Fischer-Tropsch reaction. Ethane is produced from CO and Hydrogen in the Fischer-Tropsch reaction. CO is produced from atmospheric CO2 and Hydrogen using the reverse water gas shift reaction. Hydrogen can be obtained through electrolysis of water.
The steam cracking produces acetylene, that can be recovered using a catalyst.[1]
The steam cracking process also produces benzene as a byproduct, that can be used for the production of polyester.
Uses
Ethylene is used to create polyethylene by polymerization.
Ethylene is also used to produce Ethylene glycol, a precursor to the production of polyester(PET) and an antifreeze.
Ethylene Glycol (CH2OH)2 is produced from ethylene (ethene), via the intermediate ethylene oxide (C2H4O). Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: C2H4O + H2O → HO−CH2CH2−OH
| This article is a stub. You can help Marspedia by expanding it. |