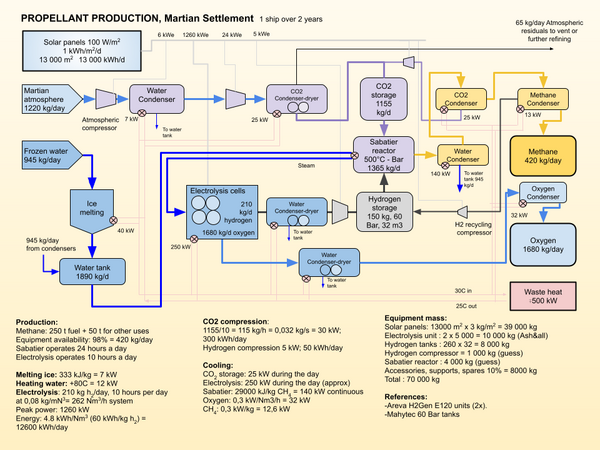
Sabatier/Water Electrolysis Process
The Sabatier reaction and water electrolysis are used to convert atmospheric carbon dioxide and water extracted from regolith, or the atmosphere, into propellant. Hydrogen shipped from Earth could also be used in certain scenarios to avoid the need for the electrolysis process[1].
The water electrolysis separates water into hydrogen and oxygen. In is by far the most energy intensive stage in the process. The oxygen is stored for later use in a vehicle propulsion system. The hydrogen is combined with atmospheric CO2 to create methane and water. The chemical reaction is the following:
- CO2 + 4H2 → CH4 + 2H2O -165 kJ/mol
The reaction takes place in the presence catalysts at a temperature between 300 and 400C. A nickel catalyst is the most likely candidate, or a catalyst made out of ruthenium or alumina might be used.
The Sabatier process produces a ratio of 4:1 of oxygen to methane, slightly more than the ratio used for propulsion, that is between 3.6 and 3.8 :1. Excess oxygen can be used for the settlement atmosphere or stored for future use.
Detailed process and alternatives
The illustration shows just one of the possible process arrangements. Compression between the Electrolysis unit and the Sabatier unit might be avoided with other choices of equipment, and the storage tanks might be replaced by metal hydride reservoirs. If dust storms are particularly bad during a synod, the production rate might be too low to complete the refueling of a transportation vehicle. Use of nuclear power rather than solar power would allow for continuous production and reduce the mass of equipment required.
The electrolysis unit uses an electrolyte, into which water is added. An electrical current between two electrodes splits the water molecules into hydrogen and oxygen, with the hydrogen migrating to one electrode and the oxygen to the other.
- A unit mass for the electrolytic cells of 4.25 kg/kh/hr has been proposed for the cells(electrolysis). This puts the cells at 340 kg for this unit. With additional structural support, cooling and drying systems (both oxygen and hydrogen are saturated with water at the outlet of the electrolysis unit) piping and controls, two 5000 kg units should be sufficient for the application.
Heat rejected from the process can be used to melt and heat the input ice, as well as heat the settlement. However, a lot of the heat is at very low temperatures and may have to be rejected into the Martian environment. A fuel production system using approximately 6-700 kW of power continuously should be capable of producing the propellant required for a SpaceX Starship vehicle over 2 years, or a Martian synod.
Another alternative is the use of the Sulfur Iodine cycle which uses thermal energy to split water into H2 and O2. This generally requires a turboinductor to create a sufficiently hot gas stream from the much lower output temperature of a standard nuclear reactor such as a KiloPower. The primary advantage of this method is that it can more directly use the nuclear energy, reducing the requirement for large scale electricity generation.
A final alternative is the use of ammonia decomposition through sodium amide. This allows for hydrogen to be stored chemically as ammonia, and then decomposed into hydrogen as needed to feed the Sabatier reactor. This also allows for biological processes, such as nitrogen fixing microbes, to provide an alternative path to hydrogen synthesis.
Sabatier reactor
A Sabatier reactor includes a catalyst, generally nickel. The general form of the reactor is tubular, to increase the contact surfaces. The process operates at high pressure (30 Bar) and temperature (300-400C). There is an interesting synergy possible in combining the Sabatier process with electrolysis. Some of heat heat from the process could be used to preheat the water for the electrolysis. High temperature electrolysis should be more efficient than electrolysis at lower temperatures.
If nickel is produced in-situ on Mars, additive printing could be used to prepare catalytic elements for the Sabatier process. The nickel might serve as well to produce electrodes for the electrolysis process.
Current developments
The Helmeth[2] program in Europe is developing a version of the Sabatier/Water electrolysis process for Power to Gas applications. The EU project HELMETH coordinated by KIT has demonstrated that high-temperature electrolysis and methanation can be combined in a power-to-gas process with an efficiency of more than 75 percent[3].
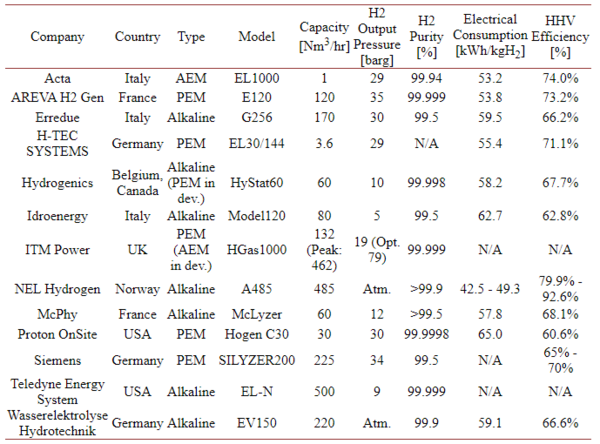
A list of current developments for hydrogen generation by electrolysis can be found here (2018)[4].
Elon Musk of SpaceX recently tweeted that they would start producing methane on Earth using the Sabatier process for their Starship launchers. https://twitter.com/elonmusk/status/1470519292651352070
See Also
References
- ↑ Compact and Lightweight Sabatier Reactor for Carbon Dioxide Reduction https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20120016419.pdf
- ↑ http://www.helmeth.eu/index.php
- ↑ http://www.helmeth.eu/images/joomlaplates/documents/PI_2018_009_Power%20to%20Gas%20with%20High%20Efficiency.pdf
- ↑ BUTTLER, Alexander et SPLIETHOFF, Hartmut. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renewable and Sustainable Energy Reviews, 2018, vol. 82, p. 2440-2454.