Difference between revisions of "Sabatier/Water Electrolysis Process"
| Line 1: | Line 1: | ||
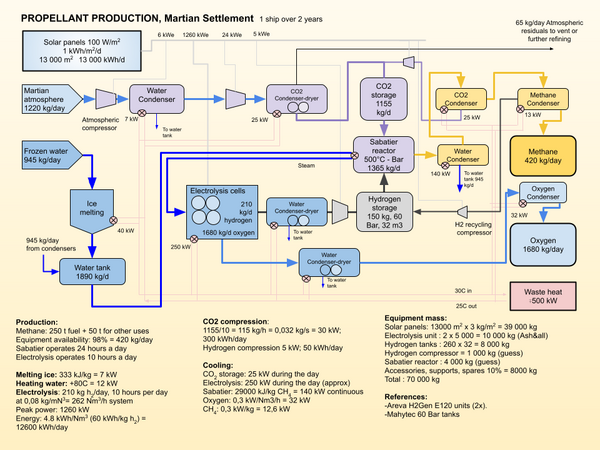
| + | [[File:Propellant production.png|thumb|600x600px|Schematic of Methane production system for a Single SpaceX Starship over a period of two years.Electrolysis and hydrogen storage are off the shelf. Sabatier reactor needs to be developed.]] | ||
The Sabatier reaction is used to convert [[Hydrogen]] shipped from [[Earth]] or [[electrolysis|processed]] from Martian [[water|water-ice]] and [[Carbon dioxide]] from the Martian atmosphere to create water and [[methane]] for use as a fuel, which can be converted to Hydrogen. This fuel will generate more thrust per unit mass than hydrogen. | The Sabatier reaction is used to convert [[Hydrogen]] shipped from [[Earth]] or [[electrolysis|processed]] from Martian [[water|water-ice]] and [[Carbon dioxide]] from the Martian atmosphere to create water and [[methane]] for use as a fuel, which can be converted to Hydrogen. This fuel will generate more thrust per unit mass than hydrogen. | ||
| − | :CO<sub>2</sub> + 4H<sub>2</sub> → CH<sub>4</sub> + 2H<sub>2</sub>O | + | :CO<sub>2</sub> + 4H<sub>2</sub> → CH<sub>4</sub> + 2H<sub>2</sub>O -165 kJ/mol |
The forward reaction takes place in the presence of high temperatures and pressures, in the presence of a [[nickel]] [[catalyst]]. Instead of nickel, a catalyst made out of [[ruthenium]] or [[alumina]] could be used. | The forward reaction takes place in the presence of high temperatures and pressures, in the presence of a [[nickel]] [[catalyst]]. Instead of nickel, a catalyst made out of [[ruthenium]] or [[alumina]] could be used. | ||
| − | == See Also == | + | The Sabatier process produces a ratio of 4:1 of oxygen to hydrogen, slightly more than the ratio used for propulsion 3.6, to 3.8:1. Excess oxygen can be used for the colony atmosphere os stored for future use. |
| − | * [[Hydrocarbon synthesis|Hydrocarbon Synthesis]] | + | |
| − | * [[Bosch process]] | + | If [[nickel]] is produced in-situ on Mars, additive printing could be used to prepare replacement electrodes for the Sabatier process |
| + | |||
| + | ==See Also== | ||
| + | |||
| + | *[[Hydrocarbon synthesis|Hydrocarbon Synthesis]] | ||
| + | *[[Bosch process]] | ||
[[Category:In-situ Resource Utilization]] | [[Category:In-situ Resource Utilization]] | ||
Revision as of 09:42, 20 July 2019
The Sabatier reaction is used to convert Hydrogen shipped from Earth or processed from Martian water-ice and Carbon dioxide from the Martian atmosphere to create water and methane for use as a fuel, which can be converted to Hydrogen. This fuel will generate more thrust per unit mass than hydrogen.
- CO2 + 4H2 → CH4 + 2H2O -165 kJ/mol
The forward reaction takes place in the presence of high temperatures and pressures, in the presence of a nickel catalyst. Instead of nickel, a catalyst made out of ruthenium or alumina could be used.
The Sabatier process produces a ratio of 4:1 of oxygen to hydrogen, slightly more than the ratio used for propulsion 3.6, to 3.8:1. Excess oxygen can be used for the colony atmosphere os stored for future use.
If nickel is produced in-situ on Mars, additive printing could be used to prepare replacement electrodes for the Sabatier process