Difference between revisions of "Ethanol"
| Line 2: | Line 2: | ||
'''Ethanol''', also known as '''ethyl alcohol''' or '''grain alcohol''', is the most common [[alcohol]]. Its formula is C<sub>2</sub>H<sub>5</sub>OH. It is clear and colorless. Ethanol is useful mixed into gasoline because it has a higher octane number than gasoline, reducing engine knocking.<ref name=":0">[https://afdc.energy.gov/fuels/ethanol.html “Ethanol.”] n.d. U.S. Department of Energy: Alternative Fuels Data Center.</ref> | '''Ethanol''', also known as '''ethyl alcohol''' or '''grain alcohol''', is the most common [[alcohol]]. Its formula is C<sub>2</sub>H<sub>5</sub>OH. It is clear and colorless. Ethanol is useful mixed into gasoline because it has a higher octane number than gasoline, reducing engine knocking.<ref name=":0">[https://afdc.energy.gov/fuels/ethanol.html “Ethanol.”] n.d. U.S. Department of Energy: Alternative Fuels Data Center.</ref> | ||
| − | It is a mild [[depressant]] when ingested | + | Ethanol is an ingredient in many beverages. It is a mild [[depressant]] when ingested. Besides its recreational use, ethanol is important to many industries and [[food preservation]]. Ethanol can be produced on Mars using [[In-situ resource utilization|In Situ Resources]]. |
| − | ==Production== | + | ==[[In-situ resource utilization|In situ Production]]== |
[[File:Process-Ethanol.png|thumb|600x600px|Ethanol production Paths]] | [[File:Process-Ethanol.png|thumb|600x600px|Ethanol production Paths]] | ||
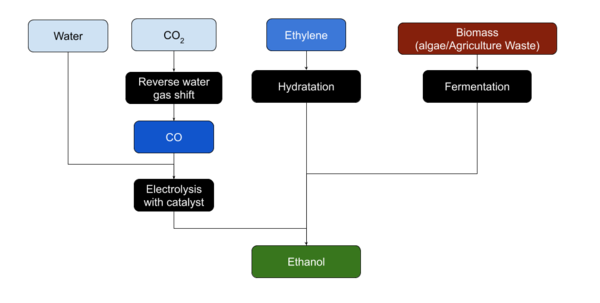
Ethanol is usually produced from [[biomass]]. Most plant material can be transformed to produce Ethanol.<ref name=":0" /> | Ethanol is usually produced from [[biomass]]. Most plant material can be transformed to produce Ethanol.<ref name=":0" /> | ||
| Line 15: | Line 15: | ||
==Uses== | ==Uses== | ||
| + | ===Recreation and nutrition=== | ||
| + | Ingestion of excessive ethanol in [[food]] or drink causes intoxication. It is a mild depressant, and impairs judgement and reduces inhibitions. Managed use of ethanol may be used to raise [[morale]]. Ethanol ingestion may be restricted heavily in a [[settlement]] due to the risk to oneself and others. | ||
===Industrial Use=== | ===Industrial Use=== | ||
Ethanol is useful as a [[fuel]]. In contrast to most other chemical [[energy]] sources (or energy vectors, in this case), ethanol is relatively non-toxic and unreactive. | Ethanol is useful as a [[fuel]]. In contrast to most other chemical [[energy]] sources (or energy vectors, in this case), ethanol is relatively non-toxic and unreactive. | ||
| Line 22: | Line 24: | ||
===Food Preservation=== | ===Food Preservation=== | ||
Ethanol is used to preserve [[fruits]] and other foods. | Ethanol is used to preserve [[fruits]] and other foods. | ||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
<references /> | <references /> | ||
[[category:Materials]] | [[category:Materials]] | ||
Revision as of 18:58, 25 May 2021
Ethanol, also known as ethyl alcohol or grain alcohol, is the most common alcohol. Its formula is C2H5OH. It is clear and colorless. Ethanol is useful mixed into gasoline because it has a higher octane number than gasoline, reducing engine knocking.[1]
Ethanol is an ingredient in many beverages. It is a mild depressant when ingested. Besides its recreational use, ethanol is important to many industries and food preservation. Ethanol can be produced on Mars using In Situ Resources.
Contents
In situ Production
Ethanol is usually produced from biomass. Most plant material can be transformed to produce Ethanol.[1]
Fermentation
Ethanol is most easily produced from sugars by yeasts through fermentation.
Industrial Production
Ethylene can be hydrated to produce Ethanol, although the reverse process, Ethylene production from Ethanol by dehydration, may be more common on Mars. Ethanol may also be produced form CO or CO2 and Water using a copper catalyst and electrolysis[2].[3]
Uses
Recreation and nutrition
Ingestion of excessive ethanol in food or drink causes intoxication. It is a mild depressant, and impairs judgement and reduces inhibitions. Managed use of ethanol may be used to raise morale. Ethanol ingestion may be restricted heavily in a settlement due to the risk to oneself and others.
Industrial Use
Ethanol is useful as a fuel. In contrast to most other chemical energy sources (or energy vectors, in this case), ethanol is relatively non-toxic and unreactive.
Ethanol is an effective solvent for many chemicals.
Food Preservation
Ethanol is used to preserve fruits and other foods.
References
- ↑ 1.0 1.1 “Ethanol.” n.d. U.S. Department of Energy: Alternative Fuels Data Center.
- ↑ Ethanol form CO and water https://www.technologyreview.com/2014/04/09/173425/a-less-resource-intensive-way-to-make-ethanol/
- ↑ 2020 upgraded process from CO2 https://scitechdaily.com/breakthrough-electrocatalyst-turns-carbon-dioxide-into-ethanol/