Difference between revisions of "Methanol"
| Line 6: | Line 6: | ||
==[[In-situ resource utilization|In Situ Production]]== | ==[[In-situ resource utilization|In Situ Production]]== | ||
Methanol can be produced on Mars from CO and H<sub>2</sub> ([[Syngas]]) | Methanol can be produced on Mars from CO and H<sub>2</sub> ([[Syngas]]) | ||
| + | |||
| + | CO + 2 H<sub>2</sub> → CH<sub>3</sub>OH (Copper-based catalyst<ref>https://doi.org/10.1016/B978-0-08-099424-6.00012-0</ref> at 200–300°C and 3.5–10 MPa, ) | ||
CO and H<sub>2</sub> can be produced from [[methane]] and [[water]]: | CO and H<sub>2</sub> can be produced from [[methane]] and [[water]]: | ||
| Line 15: | Line 17: | ||
:CO<sub>2</sub> + H<sub>2</sub> → H<sub>2</sub>O + CO | :CO<sub>2</sub> + H<sub>2</sub> → H<sub>2</sub>O + CO | ||
| − | H<sub>2</sub> | + | H<sub>2</sub> can be obtained from [[water]] electrolysis : |
:2H<sub>2</sub>O → 2H<sub>2</sub> + O<sub>2</sub> | :2H<sub>2</sub>O → 2H<sub>2</sub> + O<sub>2</sub> | ||
| − | + | ==Uses== | |
| − | |||
| − | |||
| − | |||
| − | == Uses == | ||
Methanol can be used to produce a large number of hydrocarbons and other chemicals. | Methanol can be used to produce a large number of hydrocarbons and other chemicals. | ||
When combined with [[methane]] it is a possible precursor for aromatic hydrocarbons such as [[ethane]] and [[toluene]]. | When combined with [[methane]] it is a possible precursor for aromatic hydrocarbons such as [[ethane]] and [[toluene]]. | ||
| − | == References == | + | ==References== |
[[category:Materials]] | [[category:Materials]] | ||
<references /> | <references /> | ||
Revision as of 09:35, 26 May 2021
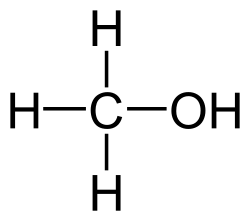
Methanol, CH3OH, is the simplest alcohol. It is commonly used as a building block for other chemicals, such as formaldehyde, acetic acid, and dimethyl ether. Like ethanol and other alcohols, methanol is toxic and highly flammable.
Methanol is a liquid carbohydrate, thus capable of storing large amounts of energy. In a settlement on Mars it has the potential to play a central part of energy management. Additionally, it can be used as a resource for making other carbohydrates, to feed methanotrophs or to produce synthetic materials.
In Situ Production
Methanol can be produced on Mars from CO and H2 (Syngas)
CO + 2 H2 → CH3OH (Copper-based catalyst[1] at 200–300°C and 3.5–10 MPa, )
CO and H2 can be produced from methane and water:
- CH4 + H2O → CO + 3 H2
CO can also be produced from CO2 via high temperature electrolysis in a MOXIE or chemically using the Bosch reaction:
- CO2 + H2 → H2O + CO
H2 can be obtained from water electrolysis :
- 2H2O → 2H2 + O2
Uses
Methanol can be used to produce a large number of hydrocarbons and other chemicals.
When combined with methane it is a possible precursor for aromatic hydrocarbons such as ethane and toluene.