Difference between revisions of "Methanol"
| Line 7: | Line 7: | ||
Methanol can be produced on Mars from CO and H<sub>2</sub> ([[Syngas]]) | Methanol can be produced on Mars from CO and H<sub>2</sub> ([[Syngas]]) | ||
| − | CO + 2 H<sub>2</sub> → CH<sub>3</sub>OH (Copper-based catalyst<ref>https://doi.org/10.1016/B978-0-08-099424-6.00012-0</ref> at 200–300°C and 3.5–10 MPa, ) | + | CO + 2 H<sub>2</sub> → CH<sub>3</sub>OH (Copper-based catalyst<ref>Methanol production : https://doi.org/10.1016/B978-0-08-099424-6.00012-0</ref> at 200–300°C and 3.5–10 MPa, ) |
CO and H<sub>2</sub> can be produced from [[methane]] and [[water]]: | CO and H<sub>2</sub> can be produced from [[methane]] and [[water]]: | ||
Revision as of 09:38, 26 May 2021
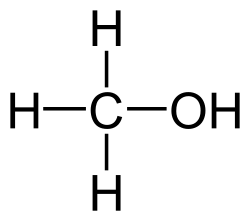
Methanol, CH3OH, is the simplest alcohol. It is commonly used as a building block for other chemicals, such as formaldehyde, acetic acid, and dimethyl ether. Like ethanol and other alcohols, methanol is toxic and highly flammable.
Methanol is a liquid carbohydrate, thus capable of storing large amounts of energy. In a settlement on Mars it has the potential to play a central part of energy management. Additionally, it can be used as a resource for making other carbohydrates, to feed methanotrophs or to produce synthetic materials.
In Situ Production
Methanol can be produced on Mars from CO and H2 (Syngas)
CO + 2 H2 → CH3OH (Copper-based catalyst[1] at 200–300°C and 3.5–10 MPa, )
CO and H2 can be produced from methane and water:
- CH4 + H2O → CO + 3 H2
CO can also be produced from CO2 via high temperature electrolysis in a MOXIE or chemically using the Bosch reaction:
- CO2 + H2 → H2O + CO
H2 can be obtained from water electrolysis :
- 2H2O → 2H2 + O2
Uses
Methanol can be used to produce a large number of hydrocarbons and other chemicals.
When combined with methane it is a possible precursor for aromatic hydrocarbons such as ethane and toluene.
References
- ↑ Methanol production : https://doi.org/10.1016/B978-0-08-099424-6.00012-0