Difference between revisions of "Ethanol"
Kee.nethery (talk | contribs) |
|||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Ethanol''', [[ | + | [[File:Ethanol-2D-flat.svg|thumb|200x200px|Ethanol molecule]] |
| + | '''Ethanol''', also known as '''ethyl alcohol''' or '''grain alcohol''', is the most common [[alcohol]]. Its formula is C<sub>2</sub>H<sub>5</sub>OH. It is clear and colorless. Ethanol is useful mixed into gasoline because it has a higher octane number than gasoline, reducing engine knocking.<ref name=":0">[https://afdc.energy.gov/fuels/ethanol.html “Ethanol.”] n.d. U.S. Department of Energy: Alternative Fuels Data Center.</ref> | ||
| + | |||
| + | Ethanol is an ingredient in many beverages. It is a mild [[depressant]] when ingested. Besides its recreational use, ethanol is important to many industries and [[food preservation]]. Ethanol can be produced on Mars using [[In-situ resource utilization|In Situ Resources]]. | ||
| + | |||
| + | ==[[In-situ resource utilization|In situ Production]]== | ||
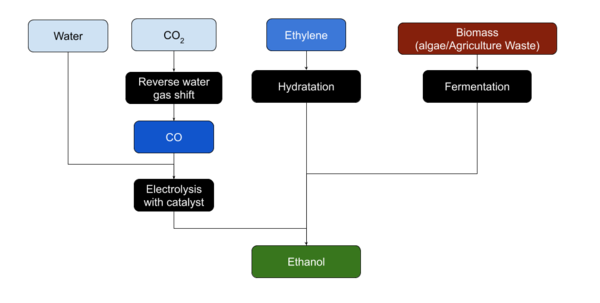
| + | [[File:Process-Ethanol.png|thumb|600x600px|Ethanol production Paths]] | ||
| + | Ethanol is usually produced from [[biomass]]. Most plant material can be transformed to produce Ethanol.<ref name=":0" /> | ||
| − | |||
===Fermentation=== | ===Fermentation=== | ||
| − | Ethanol is easily produced from [[sugars]] by [[yeast]] through [[fermentation]]. | + | Ethanol is most easily produced from [[sugars]] by [[yeast|yeasts]] through [[fermentation]]. |
| + | |||
| + | ===Industrial Production from Ethylene=== | ||
| + | [[Ethylene]] can be hydrated to produce Ethanol, although the reverse process, Ethylene production from Ethanol by dehydration, may be more common on Mars. Ethanol may also be produced form CO or CO<sub>2</sub> and Water using a copper catalyst and electrolysis<ref>Ethanol form CO and water https://www.technologyreview.com/2014/04/09/173425/a-less-resource-intensive-way-to-make-ethanol/</ref>.<ref>2020 upgraded process from CO2 https://scitechdaily.com/breakthrough-electrocatalyst-turns-carbon-dioxide-into-ethanol/</ref> | ||
| − | === | + | ===Industrial Production via Carbon Dioxide=== |
| − | + | Argonne National Laboratory in Lemont Illinois has an electrocatalyst that efficiently converts carbon dioxide into ethanol. <ref>Lina Chong (2021). Turning carbon dioxide into liquid fuel | Argonne National Laboratory. Anl.gov. Retrieved 25 November 2021, from https://www.anl.gov/article/turning-carbon-dioxide-into-liquid-fuel.</ref> | |
| − | + | "The catalyst consists of atomically dispersed copper on a carbon-powder support. By an electrochemical reaction, this catalyst breaks down CO<sub>2</sub> and water molecules and selectively reassembles the broken molecules into ethanol under an external electric field. The electrocatalytic selectivity, or 'Faradaic efficiency', of the process is over 90 percent. The catalyst also operates stably over extended operation at low voltage. The mechanism should also provide a foundation for development of highly efficient electrocatalysts for carbon dioxide conversion to a vast array of value-added chemicals." | |
| − | |||
| − | |||
| − | + | CO<sub>2</sub> is a stable molecule and transforming it into a different molecule is normally energy-intensive and costly. The electrochemical process of CO<sub>2</sub>-to-ethanol conversion using the catalyst could take advantage of the low-cost electricity available during off-peak hours. Because the process runs at low temperature and pressure, it can start and stop rapidly in response to the intermittent supply of excess electricity. | |
| − | |||
| + | ==Uses== | ||
| + | ===Recreation and nutrition=== | ||
| + | Ingestion of excessive ethanol in [[food]] or drink causes intoxication. It is a mild depressant, and impairs judgement and reduces inhibitions. Managed use of ethanol may be used to raise [[morale]]. Ethanol ingestion may be restricted heavily in a [[settlement]] due to the risk to oneself and others. | ||
===Industrial Use=== | ===Industrial Use=== | ||
| − | Ethanol is useful as a [[fuel]]. In contrast to most other chemical [[energy]] sources, ethanol is relatively non-toxic and unreactive. | + | Ethanol is useful as a [[fuel]]. In contrast to most other chemical [[energy]] sources (or energy vectors, in this case), ethanol is relatively non-toxic and unreactive. |
Ethanol is an effective [[solvent]] for many chemicals. | Ethanol is an effective [[solvent]] for many chemicals. | ||
| + | ===Food Preservation=== | ||
| + | Ethanol is used to preserve [[fruits]] and other foods. | ||
| + | ==References== | ||
| + | <references /> | ||
[[category:Materials]] | [[category:Materials]] | ||
Latest revision as of 13:46, 25 November 2021
Ethanol, also known as ethyl alcohol or grain alcohol, is the most common alcohol. Its formula is C2H5OH. It is clear and colorless. Ethanol is useful mixed into gasoline because it has a higher octane number than gasoline, reducing engine knocking.[1]
Ethanol is an ingredient in many beverages. It is a mild depressant when ingested. Besides its recreational use, ethanol is important to many industries and food preservation. Ethanol can be produced on Mars using In Situ Resources.
Contents
In situ Production
Ethanol is usually produced from biomass. Most plant material can be transformed to produce Ethanol.[1]
Fermentation
Ethanol is most easily produced from sugars by yeasts through fermentation.
Industrial Production from Ethylene
Ethylene can be hydrated to produce Ethanol, although the reverse process, Ethylene production from Ethanol by dehydration, may be more common on Mars. Ethanol may also be produced form CO or CO2 and Water using a copper catalyst and electrolysis[2].[3]
Industrial Production via Carbon Dioxide
Argonne National Laboratory in Lemont Illinois has an electrocatalyst that efficiently converts carbon dioxide into ethanol. [4]
"The catalyst consists of atomically dispersed copper on a carbon-powder support. By an electrochemical reaction, this catalyst breaks down CO2 and water molecules and selectively reassembles the broken molecules into ethanol under an external electric field. The electrocatalytic selectivity, or 'Faradaic efficiency', of the process is over 90 percent. The catalyst also operates stably over extended operation at low voltage. The mechanism should also provide a foundation for development of highly efficient electrocatalysts for carbon dioxide conversion to a vast array of value-added chemicals."
CO2 is a stable molecule and transforming it into a different molecule is normally energy-intensive and costly. The electrochemical process of CO2-to-ethanol conversion using the catalyst could take advantage of the low-cost electricity available during off-peak hours. Because the process runs at low temperature and pressure, it can start and stop rapidly in response to the intermittent supply of excess electricity.
Uses
Recreation and nutrition
Ingestion of excessive ethanol in food or drink causes intoxication. It is a mild depressant, and impairs judgement and reduces inhibitions. Managed use of ethanol may be used to raise morale. Ethanol ingestion may be restricted heavily in a settlement due to the risk to oneself and others.
Industrial Use
Ethanol is useful as a fuel. In contrast to most other chemical energy sources (or energy vectors, in this case), ethanol is relatively non-toxic and unreactive.
Ethanol is an effective solvent for many chemicals.
Food Preservation
Ethanol is used to preserve fruits and other foods.
References
- ↑ 1.0 1.1 “Ethanol.” n.d. U.S. Department of Energy: Alternative Fuels Data Center.
- ↑ Ethanol form CO and water https://www.technologyreview.com/2014/04/09/173425/a-less-resource-intensive-way-to-make-ethanol/
- ↑ 2020 upgraded process from CO2 https://scitechdaily.com/breakthrough-electrocatalyst-turns-carbon-dioxide-into-ethanol/
- ↑ Lina Chong (2021). Turning carbon dioxide into liquid fuel | Argonne National Laboratory. Anl.gov. Retrieved 25 November 2021, from https://www.anl.gov/article/turning-carbon-dioxide-into-liquid-fuel.