Difference between revisions of "Electrolysis"
| (18 intermediate revisions by the same user not shown) | |||
| Line 13: | Line 13: | ||
*[[Hydrogen]] and [[Oxygen]] | *[[Hydrogen]] and [[Oxygen]] | ||
| − | There are a number of other industrial processes which are based on the same | + | There are a number of other industrial processes which are based on the same principle. |
| − | == | + | ==Water electrolysis== |
| − | Water is | + | Water is available in large quantities on [[Mars]]. Electrolysis can be used to produce oxygen as part of the atmosphere required for an artificial [[habitat]]. The oxygen can also be used in a propulsion system. The [[hydrogen]] produced can be used for [[hydrocarbon synthesis]], yielding [[synthetic materials]] for [[space suit]]s etc. However, the main use for the electrolysis of water is likely to be propellant production. |
| − | + | Electrolytic cells for hydrogen production have an efficiency of about 70-80%. They may eventually reach [[w:Electrolysis_of_water#Efficiency|96%]]. Preheating the water increases the efficiency of the electrolysis process. Combining electrolysis with the high temperature requirements of the [[Sabatier/Water Electrolysis Process|Sabatier]] process (400°C) can improve efficiency. Using waste process heat might be an interesting alternative. | |
| − | == | + | The three main technologies under development are<ref>BUTTLER, Alexander et SPLIETHOFF, Hartmut. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. ''Renewable and Sustainable Energy Reviews'', 2018, vol. 82, p. 2440-2454.</ref>: |
| + | |||
| + | # AEL (alkaline electrolysis) | ||
| + | # PEMEL (proton exchange membrane electrolysis) | ||
| + | # SOEL solid oxide electrolysis | ||
| + | |||
| + | ===Electrolyte=== | ||
| + | An electrolyte is usually required to increase the conductivity of water and increase the reaction rate. Both acid and basic solutions are possible. A solid polymer electrolyte can also be used such as Nafion and when applied with a special catalyst on each side of the membrane can efficiently split the water molecule with as little as 1.5 volts. There are also a number of other solid electrolyte systems that have been trialed and developed with a number of electrolysis systems now available commercially that use solid electrolytes<ref>Wikipedia https://en.wikipedia.org/wiki/Electrolysis_of_water#Electrolyte_for_water_electrolysis</ref>. | ||
| + | |||
| + | ===Design parameters=== | ||
| + | Solid polymer electrolyte system for use in space have been projected with the following characteristics (ASH et all. 1981): | ||
| + | *Conversion efficiencies of approx. 90%, with cell lives approaching 40,000hr. (Heald et al. (1978)0 | ||
| + | *Specific Power 4.47 kW/kg/hr | ||
| + | *Specific Weight 4.25 kg/kg/hr | ||
| + | *Specific Volume 0.06 m3/kg/hr | ||
| + | *The reference flow rate is the mass flow rate of input water in kg/hr. | ||
| + | *These specific weight includes a mass allowance for radiant cooling of the electrolysis cell | ||
| + | |||
| + | ===Efficiency=== | ||
| + | As of 2022, commercial electrolysis requires around 53 kWh of electricity to produce one kg of hydrogen, which holds 33.6 kWh of energy, for an efficiency of 63,4%.(Wikipedia. However, a number et new processes are under development that might achieves efficiencies of 95%. | ||
| + | For example, Hysata proposes a process using capillary membrane that would reach an efficiency of 95 to 98%. https://hysata.com/our-technology/ | ||
| + | |||
| + | ==Aluminum electrolysis== | ||
| + | Aluminum electrolysis is presently accomplished using the Hall-Heroux process. Aluminum producers Alcoa and Rio Tinto, working with Apple, have announced a new process, ELYSIS, that does not produce CO2 and therefore does not require carbon anodes.<ref>https://www.alcoa.com/sustainability/en/elysis</ref> The process used proprietary anode materials to create free oxygen a the anode from the electrolytic process. | ||
| + | Rio Tinto is presently (2023) building a prototype production unit to demonstrate the viability of the technology. | ||
| + | This would be interesting on Mars as it eliminates the need for carbon, that would need to be produced from CO2, to then be consumed by the smelting process to recreate CO2. The energy needs for aluminum production would then be significantly reduced, and the oxygen would be a useful byproduct. | ||
| + | |||
| + | ==References== | ||
*[http://en.wikipedia.org/wiki/Electrolysis Wikipedia article on electrolysis] | *[http://en.wikipedia.org/wiki/Electrolysis Wikipedia article on electrolysis] | ||
| + | *{{lunarp|Water Splitting}} | ||
[[Category:In-situ Resource Utilization]] | [[Category:In-situ Resource Utilization]] | ||
| + | <references /> | ||
Latest revision as of 05:30, 11 October 2023
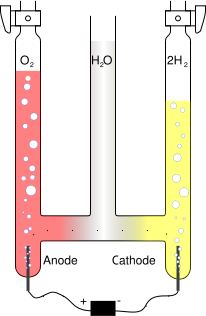
Electrolysis is the process of separating a chemical compound by passing an electrical current through it, either molten or in solution.
By applying a voltage across two electrodes immersed in the liquid, an electric field is set up, causing separation of ions in order to maintain electrical equilibrium. For instance, water can be separated into hydrogen from oxygen, with the negatively charged hydroxide ions OH- drawn to the positive electrode and the positively charged hydronium ions H3O+ drawn to the negative electrode. When arriving at the electrodes the ions are discharged and become gaseous oxygen and hydrogen respectively. The net effect of of the migration of ions is identical to the flow of an electric current through the liquid.
Electrolysis is a common industrial process, used in the production of:
There are a number of other industrial processes which are based on the same principle.
Contents
Water electrolysis
Water is available in large quantities on Mars. Electrolysis can be used to produce oxygen as part of the atmosphere required for an artificial habitat. The oxygen can also be used in a propulsion system. The hydrogen produced can be used for hydrocarbon synthesis, yielding synthetic materials for space suits etc. However, the main use for the electrolysis of water is likely to be propellant production.
Electrolytic cells for hydrogen production have an efficiency of about 70-80%. They may eventually reach 96%. Preheating the water increases the efficiency of the electrolysis process. Combining electrolysis with the high temperature requirements of the Sabatier process (400°C) can improve efficiency. Using waste process heat might be an interesting alternative.
The three main technologies under development are[1]:
- AEL (alkaline electrolysis)
- PEMEL (proton exchange membrane electrolysis)
- SOEL solid oxide electrolysis
Electrolyte
An electrolyte is usually required to increase the conductivity of water and increase the reaction rate. Both acid and basic solutions are possible. A solid polymer electrolyte can also be used such as Nafion and when applied with a special catalyst on each side of the membrane can efficiently split the water molecule with as little as 1.5 volts. There are also a number of other solid electrolyte systems that have been trialed and developed with a number of electrolysis systems now available commercially that use solid electrolytes[2].
Design parameters
Solid polymer electrolyte system for use in space have been projected with the following characteristics (ASH et all. 1981):
- Conversion efficiencies of approx. 90%, with cell lives approaching 40,000hr. (Heald et al. (1978)0
- Specific Power 4.47 kW/kg/hr
- Specific Weight 4.25 kg/kg/hr
- Specific Volume 0.06 m3/kg/hr
- The reference flow rate is the mass flow rate of input water in kg/hr.
- These specific weight includes a mass allowance for radiant cooling of the electrolysis cell
Efficiency
As of 2022, commercial electrolysis requires around 53 kWh of electricity to produce one kg of hydrogen, which holds 33.6 kWh of energy, for an efficiency of 63,4%.(Wikipedia. However, a number et new processes are under development that might achieves efficiencies of 95%. For example, Hysata proposes a process using capillary membrane that would reach an efficiency of 95 to 98%. https://hysata.com/our-technology/
Aluminum electrolysis
Aluminum electrolysis is presently accomplished using the Hall-Heroux process. Aluminum producers Alcoa and Rio Tinto, working with Apple, have announced a new process, ELYSIS, that does not produce CO2 and therefore does not require carbon anodes.[3] The process used proprietary anode materials to create free oxygen a the anode from the electrolytic process. Rio Tinto is presently (2023) building a prototype production unit to demonstrate the viability of the technology. This would be interesting on Mars as it eliminates the need for carbon, that would need to be produced from CO2, to then be consumed by the smelting process to recreate CO2. The energy needs for aluminum production would then be significantly reduced, and the oxygen would be a useful byproduct.
References
- ↑ BUTTLER, Alexander et SPLIETHOFF, Hartmut. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renewable and Sustainable Energy Reviews, 2018, vol. 82, p. 2440-2454.
- ↑ Wikipedia https://en.wikipedia.org/wiki/Electrolysis_of_water#Electrolyte_for_water_electrolysis
- ↑ https://www.alcoa.com/sustainability/en/elysis