Difference between revisions of "Water Infrastructure"
| Line 10: | Line 10: | ||
Water at freezing temperature has a hardness of about 1.5 Mohs<ref>https://en.wikipedia.org/wiki/Mohs_scale_of_mineral_hardness</ref>. This puts it between talc and gypsum, so relatively soft. However, ice gets harder as it gets colder. At -70C its hardness on the Mohs is about 6<ref>http://academic.emporia.edu/aberjame/ice/lec02/lec2.htm</ref>, or just below the hardness of quartz. So drilling through cold martian ice will be very close to drilling through granite, that has a hardness of 6 to 7. | Water at freezing temperature has a hardness of about 1.5 Mohs<ref>https://en.wikipedia.org/wiki/Mohs_scale_of_mineral_hardness</ref>. This puts it between talc and gypsum, so relatively soft. However, ice gets harder as it gets colder. At -70C its hardness on the Mohs is about 6<ref>http://academic.emporia.edu/aberjame/ice/lec02/lec2.htm</ref>, or just below the hardness of quartz. So drilling through cold martian ice will be very close to drilling through granite, that has a hardness of 6 to 7. | ||
| − | Typical grinding mill operate at about 10 kWh per tonne<ref>IMPROVING ENERGY EFFICIENCY VIA OPTIMIZED CHARGE MOTION AND SLURRY FLOW IN PLANT SCALE SAG MILLS</ref>. If the ice is still at -70C then | + | Typical grinding mill operate at about 10 kWh per tonne<ref>IMPROVING ENERGY EFFICIENCY VIA OPTIMIZED CHARGE MOTION AND SLURRY FLOW IN PLANT SCALE SAG MILLS</ref>. If the ice is still at -70C then crushing it might require up to 36 kJ/kg. This is significantly less than the power required to melt the ice, so it is likely ice will be crushed at the source to be made transportable, but melted at the settlement, ideally using waste heat. |
| − | === Transportation === | + | ===Transportation=== |
Transportation of water on Mars will probably mostly be done using heavy trucks. These use about 2,4 kJ/kg/km on Earth.<ref>https://en.wikipedia.org/wiki/Energy_efficiency_in_transport</ref>. For a water source 15 km away, the energy to move the ice will be similar to the energy to extract the ice, but still less than 5% of the energy required to melt the ice. Rail might reduce the energy significantly, and the lower martian gravity might help as well. | Transportation of water on Mars will probably mostly be done using heavy trucks. These use about 2,4 kJ/kg/km on Earth.<ref>https://en.wikipedia.org/wiki/Energy_efficiency_in_transport</ref>. For a water source 15 km away, the energy to move the ice will be similar to the energy to extract the ice, but still less than 5% of the energy required to melt the ice. Rail might reduce the energy significantly, and the lower martian gravity might help as well. | ||
Revision as of 15:23, 23 December 2019
Water, on Mars as on Earth, is essential to life and therefore to any Mars settlement. Water infrastructures, including water treatment facilities and water distribution systems will be part of any Mars settlement from day one. Indeed it will be part of the vehicles bringing the settlers to Mars.
Contents
Water extraction
Water will be available from a number of sources on Mars. However, it is not yet clear if the water will contain contaminants, and what will be the nature of these.
Melting
Water will mostly be in a frozen state and will need to be heated to be melted. This required about 336 kJ per kg for the phase change from ice to water. Heating the water from Mars ambient temperature to the water treatment process temperature will also require about 4,18 kJ/kg x 70C = 292 kJ/kg for a total of 628 kJ/kg.
Crushing and milling
Water at freezing temperature has a hardness of about 1.5 Mohs[1]. This puts it between talc and gypsum, so relatively soft. However, ice gets harder as it gets colder. At -70C its hardness on the Mohs is about 6[2], or just below the hardness of quartz. So drilling through cold martian ice will be very close to drilling through granite, that has a hardness of 6 to 7.
Typical grinding mill operate at about 10 kWh per tonne[3]. If the ice is still at -70C then crushing it might require up to 36 kJ/kg. This is significantly less than the power required to melt the ice, so it is likely ice will be crushed at the source to be made transportable, but melted at the settlement, ideally using waste heat.
Transportation
Transportation of water on Mars will probably mostly be done using heavy trucks. These use about 2,4 kJ/kg/km on Earth.[4]. For a water source 15 km away, the energy to move the ice will be similar to the energy to extract the ice, but still less than 5% of the energy required to melt the ice. Rail might reduce the energy significantly, and the lower martian gravity might help as well.
Recycled water will incur none of the above costs. So water treatment is interesting as an energy efficiency measure at the settlement.
Water treatment plants
Extracted water may contain contaminants such as dust, salts and other materials
Potable water may need biological treatment (chlorine).
Water may (will) be mixed with re-used water. There will be large amounts of water removed by dehumidification from greenhouses.
Water storage and distribution
Water is usually stored in tanks and distributed through pipes using pumps. Raw water is water that has been extracted, but not yet treated for use.
Water production
Water will be produced from two sources in a settlement. 1- Respiration and biological processes produce water through combustion of food in the organism. 2- Propellant production produces water as part of the Sabatier process output. For every methane molecule produced, four water molecules are required, and two water molecules are returned. 4H2O+CO2=2H2O+O2+CH4.
As production increases in a growing settlement, water may become an output from the fabrication of steel if a hydrogen reduction system is used.
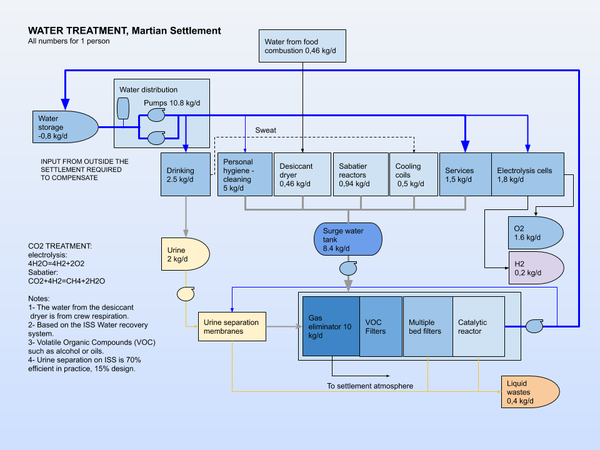
Waste water treatment
Used water goes to the waste water treatment plant. It is purified using different treatments and the minerals and contaminants removed are recycled. The water is stored, or mixed with water from the settlement systems and returned to water treatment plant for reuse.
References
- ↑ https://en.wikipedia.org/wiki/Mohs_scale_of_mineral_hardness
- ↑ http://academic.emporia.edu/aberjame/ice/lec02/lec2.htm
- ↑ IMPROVING ENERGY EFFICIENCY VIA OPTIMIZED CHARGE MOTION AND SLURRY FLOW IN PLANT SCALE SAG MILLS
- ↑ https://en.wikipedia.org/wiki/Energy_efficiency_in_transport