Difference between revisions of "Propellant production"
| Line 2: | Line 2: | ||
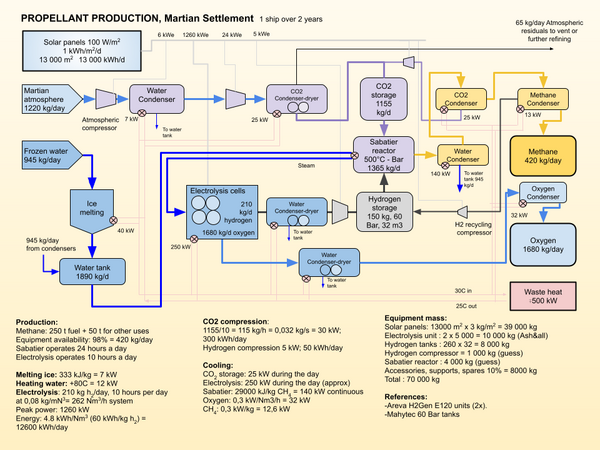
Propellant production for a Martian settlement will probably use water from the [[regolith]] and [[CO2]] from the atmosphere to produce [[Methane]] and [[oxygen]]. | Propellant production for a Martian settlement will probably use water from the [[regolith]] and [[CO2]] from the atmosphere to produce [[Methane]] and [[oxygen]]. | ||
| − | [[Electrolysis]] is used to produce hydrogen and oxygen. The oxygen is stored for use as a propellant. The hydrogen is combined with CO2 to produce methane and water using the [[Sabatier process|Sabatier]] process. The water is stored for reuse, and the methane as the chemical | + | [[Electrolysis]] is used to produce hydrogen and oxygen. The oxygen is stored for use as a propellant. The hydrogen is combined with CO2 to produce methane and water using the [[Sabatier process|Sabatier]] process. The water is stored for reuse, and the methane as the chemical fuel for the Methane-Oxygen (Methalox) propulsion system. |
| − | The image illustrates the process to create the propellant | + | The image illustrates the process required to create the propellant for a large SpaceX Starship type vehicle over a period of two years ( an Earth Mars Synod). |
Revision as of 07:28, 4 November 2020
Propellant production for a Martian settlement will probably use water from the regolith and CO2 from the atmosphere to produce Methane and oxygen.
Electrolysis is used to produce hydrogen and oxygen. The oxygen is stored for use as a propellant. The hydrogen is combined with CO2 to produce methane and water using the Sabatier process. The water is stored for reuse, and the methane as the chemical fuel for the Methane-Oxygen (Methalox) propulsion system.
The image illustrates the process required to create the propellant for a large SpaceX Starship type vehicle over a period of two years ( an Earth Mars Synod).