Difference between revisions of "Atmospheric processing"
m (→Oxygen) |
m (→Nitrogen) |
||
| Line 39: | Line 39: | ||
The balance of the remaining gas after carbon dioxide condensation contains mostly [[nitrogen]] and [[argon]]. This mixture can serve as a buffer for oxygen to produce a breathable atmosphere. Remaining traces of Carbon monoxide must be removed if the gas is to be used for the settlement atmosphere. | The balance of the remaining gas after carbon dioxide condensation contains mostly [[nitrogen]] and [[argon]]. This mixture can serve as a buffer for oxygen to produce a breathable atmosphere. Remaining traces of Carbon monoxide must be removed if the gas is to be used for the settlement atmosphere. | ||
| − | [[Nitrogen]] can be used to create [[ammonia]] and nitrates in [[fertilizer]] and as nitric acid in industry. | + | [[Nitrogen]] can be used to create [[ammonia]] and nitrates in [[fertilizer]] and as nitric acid in industry. A process such as an optimized low pressure Haber reactor <ref>https://www.ammoniaenergy.org/wp-content/uploads/2019/11/20191112.1043-Ammonia-Talk-NH3-Fuel.pdf</ref> would allow for simplified industrial production, and the use of nitrogen fixing microbes would allow for the biological production of both ammonia and nitrates. |
===Argon=== | ===Argon=== | ||
Revision as of 04:43, 23 July 2020
Atmospheric processing describes the extraction of substances out of the Martian atmosphere and the usage as raw material for further processing. Unlike surface and sub-surface mining, the atmospheric mining does not require the movement of large amounts of regolith or rock with heavy machinery, nor is expensive transport per rover or railroad necessary. The atmosphere can simply be sucked in through a pipe at every location, and the processing is done inside of buildings. Also, the maintenance of all the atmospheric mining machinery could be in-house, which would be a major safety advantage.
Collection of Atmosphere
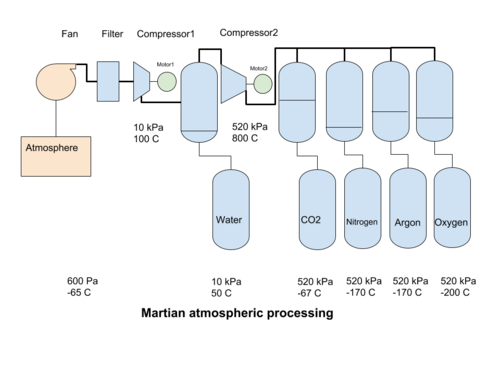
There are a number of ways the martian atmosphere can be processed to separate it into its individual components for process use. Mechanical compression is one of the main possibilities for large scale operations
In mechanical compression systems, a fan collects martian atmosphere and passes it through filters to separate out the dust. Then a compressor increases the pressure of the atmosphere to reach a point when water can be condensed out. Then a second compressor increases the pressure to the liquefaction point of CO2. The liquid CO2 is removed, and the leftover gases can be cooled further, to condense out into their liquid phases. Alternatively, using ionic liquids[1], the CO2 can be removed at atmospheric pressure, requiring only the N2 and Ar fractions to be compressed.
Process
Filtering
The martian atmosphere contains 1,8e-7 kg of dust per m3, on average[2]. This dust must be removed to avoid process problems in subsequent steps. Dust collectors or electrostatic precipitation will be required.
Compression
Compression from the initial atmospheric pressure of 600 Pa to 0,1 bar (10 kPa, or 1/10 Earth atmosphere) heats the atmosphere to 100°C but also creates adequate conditions for the condensation of water when it is cooled to about 40°C. It can then be collected at the bottom of a pressure vessel and removed. A second compressor increases the gas pressure to a bit above 5,1 bar (520 kPa) but also boosts the temperature to over 800°C. 520 kPa is the lowest pressure at which CO2 can change from a gas to a liquid (at lower pressures it turns directly into a solid), at about -57°C. The liquid carbon dioxide can then be separated by gravity from the other gasses. The total compression ratio from 600 to 520 000 Pa is about 860 times.
An exit temperature for a compressor of 800°C is extremely high. Intermediate cooling with isobaric compression (change of volume with constant pressure) will be required to reduce the exit temperatures of the compressors.
Condensation
The change from a gas phase to a liquid phase is condensation. The compressors create a pressure environment where cooling the gas condensates one of the fractions of the atmosphere. The first gas to condensate out is water, at 40°C for 10 kPa pressure. After an increase in pressure, the hot dry gas is cooled further to condense the CO2 , at -57°C for 520 kPa. The condensed liquid CO2 can be removed by gravity. Nitrogen at 510 kPa will liquefy at -170°C, and Argon at about the same temperature, so care must be taken if we want to keep these two gases separate. The next gas to condense out is Oxygen. All of the cooled gas may be used for process purposes, or some of the gases may be released back into the atmosphere. The expansion of the gas back to atmospheric pressure produces a cooling effect that can be used in the process to help condensate out the atmospheric gases.
Desiccation (adsorption) and scrubbers
Desiccation might be used as an alternative to compression for water removal. A desiccant bed, or a desiccant coated wheel, can be inserted into the gas stream, after filtration. The desiccant captures the water, that is later removed from the desiccant using heat in a regenerative process. This might use the heat from the secondary compression, that is significant at 520 kPa, rather than mechanical work. Similar systems can be used for CO2, N2 and Argon separation as well. For example, amines are used to remove CO2 from the atmosphere of submarines[3] and of the ISS. Various types of carbon dioxide scrubbers exist or are under development[4]. Adsorption of nitrogen is an existing industrial process that should use less energy than compression/condensation systems. Ionic liquids[5] are an interesting development in scrubbers, as unlike amine scrubbers they have no vapor pressure. As such they can extract CO2 from the Martian atmosphere at atmospheric pressure. This then makes compression more efficient, as it's only compressing the N2 and Ar fractions.
Process outputs
Dust
The Martian atmosphere contains variable amounts of dust, which consists of minerals lifted by wind from the surface regolith. Electrostatic filters, cyclonic separators or dust collectors with fabric filters will be used to remove the dust to prevent it from damaging the equipment.
Water
The 0.03 % water vapor (H2O) is equivalent to about 10 % air humidity after adiabatic compression and cooling to around 1°C. A device similar to an air dehumidifier can be used to extract this water.
Carbon Dioxide
96% of the Martian atmosphere is Carbon dioxide. It can be used for hydrocarbon synthesis, including the production of methane based fuel. It can also be used to produce Oxygen through splitting of the H2O produced during hydrocarbon synthesis, or during the production of Carbon through the Bosch process. Alternatively, it can also be electrolysed in a MOXIE, producing CO and O2 or used in farming.
Nitrogen
The balance of the remaining gas after carbon dioxide condensation contains mostly nitrogen and argon. This mixture can serve as a buffer for oxygen to produce a breathable atmosphere. Remaining traces of Carbon monoxide must be removed if the gas is to be used for the settlement atmosphere.
Nitrogen can be used to create ammonia and nitrates in fertilizer and as nitric acid in industry. A process such as an optimized low pressure Haber reactor [6] would allow for simplified industrial production, and the use of nitrogen fixing microbes would allow for the biological production of both ammonia and nitrates.
Argon
Argon is useful for industrial processes that must be performed in an inert atmosphere. It can also be used for electric rocket propulsion. It may be used as a complement for nitrogen in a martian settlement if it proves to be safe for that purpose.
Oxygen
Oxygen is used for the creation of the settlement atmosphere or for a large number of industrial processes. Oxygen can be produced through the splitting of water directly, via CO2 chemically, or via photosynthesis. A chemical method for the production of O2 from CO2 is:
- CO2 + H2 → CO + H2O (The Reverse Water-Gas Shift Reaction)
- 2H2O → 2H2 + O2
Where the H2 is recycled from reaction 2 to reaction 1, forming a loop, resulting in an overall reaction equivalent to:
- 2CO2 → 2CO + O2
The splitting of water may be obtained either through electrolysis, or thermochemically with something like the Sulfur/Iodine cycle[7].
See also
References
- ↑ https://pubs.acs.org/doi/full/10.1021/ja017593d
- ↑ https://pdfs.semanticscholar.org/418a/88f31b87f3d615a1f6116d31a078cfde8802.pdf
- ↑ http://web.mit.edu/12.000/www/m2005/a2/8/pdf1.pdf
- ↑ https://en.wikipedia.org/wiki/Carbon_dioxide_scrubber
- ↑ https://pubs.acs.org/doi/full/10.1021/ja017593d
- ↑ https://www.ammoniaenergy.org/wp-content/uploads/2019/11/20191112.1043-Ammonia-Talk-NH3-Fuel.pdf
- ↑ https://doi.org/10.1016/j.enconman.2006.02.010