Difference between revisions of "Carbon dioxide"
m (→Concentration: improved clarity of sentence) |
(→In situ Production: Mars likely formed more sulphates rather than carbonates.) |
||
| Line 29: | Line 29: | ||
==[[In-situ resource utilization|In situ Production]]== | ==[[In-situ resource utilization|In situ Production]]== | ||
| − | CO2 will be extracted [[In-situ resource utilization|in-situ]] by [[atmospheric processing]], or from carbonate rocks to provide larger industrial quantities to feed industry<ref>https://www.nature.com/articles/ngeo971</ref> Carbonate rocks are common on Earth, but seem to be rarer on Mars. It is | + | CO2 will be extracted [[In-situ resource utilization|in-situ]] by [[atmospheric processing]], or from carbonate rocks to provide larger industrial quantities to feed industry<ref>https://www.nature.com/articles/ngeo971</ref> Carbonate rocks are common on Earth, but seem to be rarer on Mars. It is now thought that Martian water was acidic, which would favour the creation of sulphates. |
It is very likely that large amounts of CO2 will be found in local clays which will out-gas if the clay is warmed. (Some clays can hold 9% of their mass in CO2 when cold.) | It is very likely that large amounts of CO2 will be found in local clays which will out-gas if the clay is warmed. (Some clays can hold 9% of their mass in CO2 when cold.) | ||
Revision as of 01:23, 30 August 2024
Carbon dioxide[1] (chemical formula: CO2) is a chemical substance that occupies about 96 % of the Martian atmosphere.
Molar Mass of 12(C)+32(O2)=44
Biological significance
The metabolism of human beings, animals and various microbes depends on the oxidation of carbohydrates, resulting in carbon dioxide and water exhalation. Plants use the carbon from carbon dioxide to produce carbohydrates and release the oxygen back to the atmosphere, completing the cycle.
- The reaction is: CO2(carbon dioxide) + 2H2O (water) + photons (light energy) → C(n)H2O(m) (carbohydrate) + O2(oxygen)+ H2O (water)[2]
Humans require that carbon dioxide levels be low in the air, so that CO2 in the blood can diffuse outwards into the air in the lungs. There can be plenty of oxygen in the air, but if CO2 goes much over 0.5% of the air pressure, then flow of CO2 out of the body is slowed. OSHA (Occupational Safety & Health Administration) has established a permissible Exposure Limit of 5,000 parts per million (ppm) or 0.5% of CO2 in the air, averaged over an 8 hour work day. At 10,000 ppm (1.0%) CO2 may cause drowsiness. At 4% to 5% it is immediately dangerous to life or health (gasping for breath, dizziness, confusion, headaches, shortness of breath). At 80,000 ppm (8% of the air pressure) it will cause unconsciousness and then death. These responses vary by individual (depending on how healthy they are), and on the length of the exposure. The treatment for too high CO2 concentration is to immediately move the subject to an area with low CO2, to allow the dangerous CO2 level in the blood to drop.
Settlement atmosphere
Carbon dioxide is required in the settlement atmosphere for plant metabolism. Standard concentration on Earth is increasing, so the value is a moving target. However, a concentration between 300 ppm (0,03%) and 1,000 ppm (0,1%) is considered acceptable[3]. Nuclear submarines have varying carbon dioxide levels that can reach 9000 ppm in normal operations, but average between 3-4000ppm[4]. A CO2 enriched environment may be beneficial for the growth of plants in greenhouses or photobioreactors.
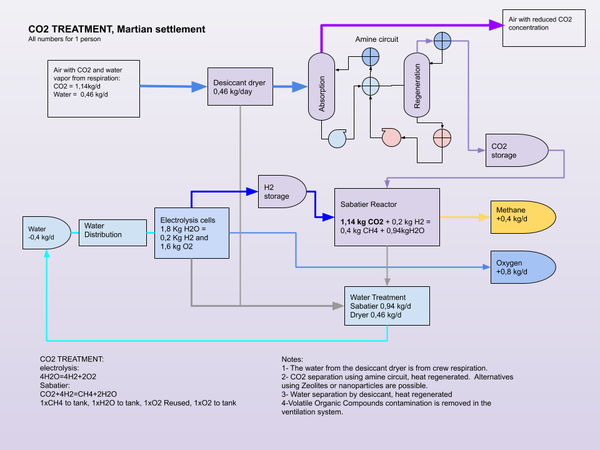
The Sabatier process can be used in place of photosynthesis to complete the atmospheric part of the carbon cycle. Methanotrophic synthesis of carbohydrates from methane would be required to complete the carbon metabolic cycle without the use of plants. Or food can be supplied from Earth or Mars for a partial cycle, where Methane from the Sabatier process can be stored for use as a propellant.
How Submarines remove carbon dioxide [5]
Submarines are a fairly good analog for a Mars habitat, a sealed system that has to manufacture oxygen and remove carbon dioxide.
Submarines have two processes for removing CO2, primary extractor is powered and has no consumables, the secondary is low tech but has consumables (typically used when the primary is undergoing maintenance or repair).
Primary uses Monoethanolamine or MEA. MEA is sprayed on the air to increase the MEA surface area with the air. MEA absorbs CO2 from the air. CO2 rich MEA gets pumped into a heater. The MEA is boiled at a high pressure which causes the CO2 to distill out first. CO2 is compressed and pushed out of the boat into the sea. Submariner spouses complain about the MEA smell from the uniforms when they return home. The MEA smell permeates everything.
Secondary CO2 scrubber uses Lithium Hydroxide LiOH. Lithium Hydroxide absorbs CO2 but in a submarine it’s a one use process. The canisters need to remain sealed until they are going to be used. LiOH powder can be sprinkled around on surfaces to absorb CO2. This is the same technology used for CO2 removal in spacesuits.
2LiOH(s) + CO2(g) -> Li2CO3(s) + H2O(g) [6]
Although the reaction can be reversed, neither the ISS nor submarines do so.
In situ Production
CO2 will be extracted in-situ by atmospheric processing, or from carbonate rocks to provide larger industrial quantities to feed industry[7] Carbonate rocks are common on Earth, but seem to be rarer on Mars. It is now thought that Martian water was acidic, which would favour the creation of sulphates.
It is very likely that large amounts of CO2 will be found in local clays which will out-gas if the clay is warmed. (Some clays can hold 9% of their mass in CO2 when cold.)
Notes on Atmosphere
CO2 forms clouds in the Martian atmosphere, which is rare. (Most planetary atmospheres do not form clouds of its primary constituent.) Mars is so cold that CO2 freezes out in the winter, causing the planet's air pressure to fall by as much as 30%. See Atmosphere for more details.
CO2 is a Greenhouse gas and if more carbon dioxide could be outgassed from the crust somehow, the planet would warm. See Terraforming for more details.
Notes at Poles
In the short northern winter CO2 will frost out. In the longer southern winter large masses of CO2 will frost or snow out of the atmosphere significantly lowering the planet's atmospheric pressure. The study, "Carbon Dioxide Ice Glaciers at the South Pole of Mars" suggests that the CO2 at the South Pole is much deeper than previously thought, being over 1 km thick in basins. [8] This is a much larger amount of CO2 trapped at the poles than previously thought.
Concentration
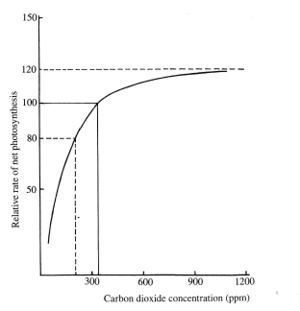
Concentration of CO2 on Earth is was 275 parts per million (ppm) in pre-industrial times. Currently it has risen above 400 ppm. Increasing concentration improves plant growth rates, however the effect is non linear and reaches a peak of 20% improvement in yields at about 1,200 ppm.[9] This may also be affected by pressure, if low pressure greenhouse are developed for Mars.
Uses
- Photosynthesis by plants in greenhouses to create carbohydrates for plant metabolism.
- Synthetic materials, hydrocarbons using the Fischer Tropsch reaction process.
- Propellant production. Methane (CH4) and Oxygen (O2), through ISRU using the Sabatier process. The hydrogen comes from Electrolysis of water or is brought from Earth.
- Carbon using the Bosch reaction process. The Bosch reaction consumes hydrogen to produce carbon and water. The hydrogen can come from electrolysis of water.
References
- ↑ https://en.wikipedia.org/wiki/Carbon_dioxide
- ↑ Photosynthesis- https://en.wikipedia.org/wiki/Photosynthesis
- ↑ Carbon dioxyde concentrationshttps://www.nap.edu/read/11170/chapter/5
- ↑ High CO2 exposures: https://www.nap.edu/read/11170/chapter/5#47
- ↑ Destin Sandlin (2021). How Do Nuclear Submarines Make Oxygen?- Smarter Every Day 251 [Video]. https://www.youtube.com/watch?v=g3Ud6mHdhlQ; SmarterEveryDay.
- ↑ Carbon Dioxide Removal – Thermodynamics. Nasa.gov. Retrieved 16 November 2021, from https://www.nasa.gov/pdf/519347main_AP_ST_CO2Removal_Therm.pdf.
- ↑ https://www.nature.com/articles/ngeo971
- ↑ https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/2022JE007193
- ↑ University of California, Agriculture and Natural ressources https://ucanr.edu/blogs/NurseryFlower/