Carbon dioxide
Carbon dioxide[1] (chemical formula: CO2) is a chemical substance that occupies about 96 % of the Martian atmosphere.
Molar Mass of 12(C)+32(O2)=44
Biological significance
The metabolism of human beings, animals and various microbes depends on the oxidation of carbohydrates, resulting in carbon dioxide and water exhalation. Plants use the carbon from carbon dioxide to produce carbohydrates and release the oxygen back to the atmosphere, completing the cycle.
- The reaction is: CO2(carbon dioxide) + 2H2O (water) + photons (light energy) → C(n)H2O(m) (carbohydrate) + O2(oxygen)+ H2O (water)[2]
Settlement atmosphere
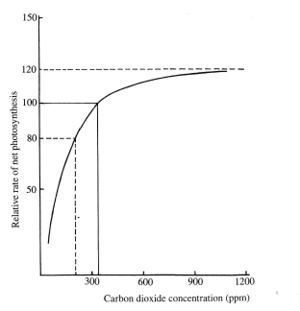
Carbon dioxide is required in the settlement atmosphere for plant metabolism. Standard concentration on Earth is increasing, so the value is a moving target. However, a concentration between 300 ppm (0,03%) and 1,000 ppm (0,1%) is considered acceptable[3]. Nuclear submarines have varying carbon dioxide levels that can reach 900 ppm in normal operations. A CO2 enriched environment may be beneficial for the growth of plants in greenhouses or photobioreactors.
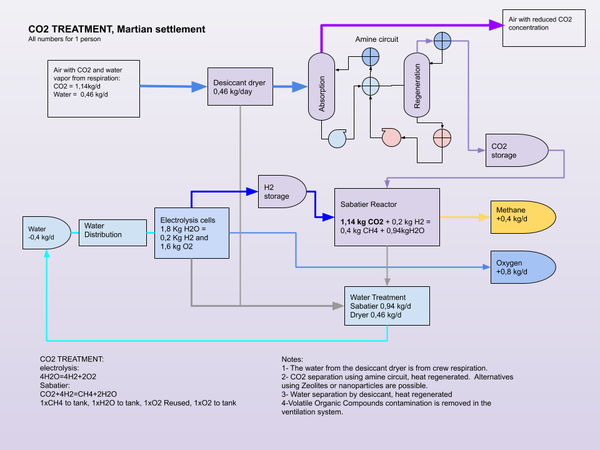
The Sabatier process can be used in place of photosynthesis to complete the atmospheric part of the carbon cycle. Methanotrophic synthesis of carbohydrates from methane would be required to complete the carbon metabolic cycle without the use of plants. Or food can be supplied from Earth or Mars for a partial cycle, where Methane from the Sabatier process can be stored for use as a propellant.
In situ Production
CO2 will be extracted in-situ by atmospheric processing, or from carbonate rocks to provide larger industrial quantities to feed industry[4] Carbonate rocks are common on Earth, but seem to be rarer on Mars. It is possible that Mars has more carbonate rock formations which are buried.
It is very likely that large amounts of CO2 will be found in local clays which will out-gas if the clay is warmed. (Some clays can hold 9% of their mass in CO2 when cold.)
Notes on Atmosphere
CO2 forms clouds in the Martian atmosphere, which is rare. (Most planets do not form clouds of its primary constituent.) Mars is so cold that CO2 freezes out in the winter, causing the planet's air pressure to fall by as much as 30%. See Atmosphere for more details.
CO2 is a Greenhouse gas and if more carbon dioxide could be outgassed from the crust somehow, the planet would warm. See Terraforming for more details.
Concentration
Concentration of CO2 on Earth is was 275 parts per million (ppm) in pre-industrial times. Currently it has risen above 400 ppm. Increasing concentration improves plant production rates, however the effect is non linear and reaches a peak of 20% improvement in yields at about 1,200 ppm.[5]
Uses
- Photosynthesis by plants in greenhouses to create carbohydrates for plant metabolism.
- Synthetic materials, hydrocarbons using the Fischer Tropsch reaction process.
- Propellant production. Methane (CH4) and Oxygen (O2), through ISRU using the Sabatier process. The hydrogen comes from Electrolysis of water or is brought from Earth.
- Carbon using the Bosch reaction process. The Bosch reaction consumes hydrogen to produce carbon and water. The hydrogen can come from electrolysis of water.
References
- ↑ https://en.wikipedia.org/wiki/Carbon_dioxide
- ↑ Photosynthesis- https://en.wikipedia.org/wiki/Photosynthesis
- ↑ Carbon dioxyde concentrationshttps://www.nap.edu/read/11170/chapter/5
- ↑ https://www.nature.com/articles/ngeo971
- ↑ University of California, Agriculture and Natural ressources https://ucanr.edu/blogs/NurseryFlower/