Difference between revisions of "Surface composition"
Jarogers2001 (talk | contribs) (→Overview: exodictionary link to andesitic) |
m (→Notes: Fixed formatting) |
||
| (15 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | This page summarized the soil composition of Mars. | |
| − | A predominant feature of the Martian surface is the [[iron oxide]]-rich dust known as [[regolith]], giving the planet its characteristic red color. This dust is very fine and the result of years of [[meteorite]] impacts pulverizing the Martian surface spreading dust around the planet. This dust is blown globally by storms, creating massive seasonal [[dust storms]] that can last for months. | + | ==Overview== |
| + | A predominant feature of the [[Mars|Martian]] surface is the [[iron|iron oxide]]-rich dust known as [[regolith]], giving the planet its characteristic red color. This dust is very fine and the result of years of [[meteorites|meteorite]] impacts pulverizing the Martian surface spreading dust around the planet. This dust is blown globally by storms, creating massive seasonal [[dust storms]] that can last for months. | ||
| − | Rock formations on the surface | + | Rock formations on the surface are primarily composed of basalt - a consequence of the extensive lava flows that once existed as a result of ancient geological activity. Analysis of soil samples collected by the [[GFDL:Viking_1|Viking landers]] in 1976 indicate iron-rich clays consistent with weathering of basaltic rocks. |
[[Image:Erebus_360.jpg|thumb|right|400px|Panorama taken on the rim of Erebus crater]] | [[Image:Erebus_360.jpg|thumb|right|400px|Panorama taken on the rim of Erebus crater]] | ||
| − | There is also evidence the Martian surface may be more silica-rich than the basalt created by lava flows, similar to [[exd:andesitic|andesitic]] rocks found on Earth (rock which crystallizes from silicate minerals at intermediate temperatures). | + | There is also evidence the Martian surface may be more silica-rich than the basalt created by lava flows, similar to [[exd:andesitic|andesitic]] rocks found on [[Earth]] (rock which crystallizes from silicate minerals at intermediate temperatures). |
| − | Mars has twice as much iron oxide in its outer layers as Earth does, despite their similar origin (meteorite impacts). This is due to the geologically active (and hotter) Earth transporting much of the surface iron deep below the terrestrial surface. Mars does not have this geological advantage to produce heat, so the iron remains in the Martian regolith, giving Mars its red | + | Mars has twice as much iron oxide in its outer layers as Earth does, despite their similar origin (meteorite impacts). This is due to the geologically active (and hotter) Earth transporting much of the surface iron deep below the terrestrial surface. Mars does not have this geological advantage to produce heat, so the iron remains in the Martian regolith, giving Mars its red color. |
| + | ==Materials== | ||
| + | Chemical composition of the soils on Mars is based upon the various data we have to date from Mars landers as well as SNC-meteorites believed to be from Mars. Different sites and sources contain different concentrations. | ||
| + | ===Spirit, Opportunity, Curiosity Rovers<ref>jpl.nasa.gov. 2012. Inspecting Soils Across Mars. [online] Available at: <https://www.jpl.nasa.gov/images/inspecting-soils-across-mars> [Accessed 18 October 2021].</ref>=== | ||
| + | Values estimated from a graph. Measurements provided by their Alpha Particle X-ray Spectrometer (APXS). Spirit data taken from 59 samples at Gusev Crater, Opportunity data taken from 23 samples at Meridiani Planum, Curiosity data taken from a sample inside a rover wheel scuff at Gale Crater. | ||
| + | {| | ||
| + | | | ||
| + | | | ||
| + | |Spirit | ||
| + | |Opportunity | ||
| + | |Curiosity | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |- | ||
| + | |SiO<sub>2</sub> | ||
| + | |Silicon Dioxide | ||
| + | |46.0 | ||
| + | |45.3 | ||
| + | |43.6 | ||
| + | |- | ||
| + | |FeO | ||
| + | |Iron(II) Oxide | ||
| + | |16.0 | ||
| + | |18.8 | ||
| + | |21.3 | ||
| + | |- | ||
| + | |Al<sub>2</sub>O<sub>3</sub> | ||
| + | |Aluminum Oxide | ||
| + | |10.2 | ||
| + | |9.16 | ||
| + | |9.58 | ||
| + | |- | ||
| + | |MgO | ||
| + | |Magnesium Oxide | ||
| + | |8.61 | ||
| + | |7.39 | ||
| + | |6.55 | ||
| + | |- | ||
| + | |CaO | ||
| + | |Calcium Oxide | ||
| + | |6.27 | ||
| + | |6.93 | ||
| + | |7.39 | ||
| + | |- | ||
| + | |SO<sub>3</sub> | ||
| + | |Sulfur Trioxide | ||
| + | |6.13 | ||
| + | |5.92 | ||
| + | |5.16 | ||
| + | |- | ||
| + | |Na<sub>2</sub>O | ||
| + | |Sodium Oxide | ||
| + | |3.00 | ||
| + | |2.20 | ||
| + | |2.20 | ||
| + | |- | ||
| + | |P<sub>2</sub>O<sub>5</sub> | ||
| + | |Phosphorus Pentoxide | ||
| + | |0.91 | ||
| + | |0.84 | ||
| + | |0.56 | ||
| + | |- | ||
| + | |TiO<sub>2</sub> | ||
| + | |Titanium Dioxide | ||
| + | |0.91 | ||
| + | |1.05 | ||
| + | |1.53 | ||
| + | |- | ||
| + | |Cl | ||
| + | |Chlorine | ||
| + | |0.70 | ||
| + | |0.64 | ||
| + | |0.63 | ||
| + | |- | ||
| + | |K<sub>2</sub>O | ||
| + | |Potassium Oxide | ||
| + | |0.42 | ||
| + | |0.49 | ||
| + | |0.63 | ||
| + | |- | ||
| + | |Cr<sub>2</sub>O<sub>3</sub> | ||
| + | |Chromium(III) Oxide | ||
| + | |0.35 | ||
| + | |0.42 | ||
| + | |0.42 | ||
| + | |- | ||
| + | |MnO | ||
| + | |Manganese(II) Oxide | ||
| + | |0.28 | ||
| + | |0.35 | ||
| + | |0.42 | ||
| + | |- | ||
| + | |Ni | ||
| + | |Nickel | ||
| + | |0.047 | ||
| + | |0.045 | ||
| + | |0.035 | ||
| + | |- | ||
| + | |Zn | ||
| + | |Zinc | ||
| + | |0.027 | ||
| + | |0.033 | ||
| + | |0.027 | ||
| + | |- | ||
| + | |Br | ||
| + | |Bromine | ||
| + | |0.005 | ||
| + | |0.008 | ||
| + | |0.003 | ||
| + | |} | ||
| − | [[category: | + | [[File:Jpl nasa gov jpegPIA16572.width-1280.jpg]] |
| + | Note: In the image SiO<sub>2</sub> and FeO values were divided by 10 to not overrun the boundaries of the image, and Ni, Zn, and Br were multiplied by 100 to be visible. | ||
| + | |||
| + | ===Viking Lander 1 & 2, Pathfinder, JSC Martian Soil Simulant<ref>Allen, C., Jager, K., Morris, R., Lindstrom, D., Lindstrom, M., & Lockwood, J. (1998). Martian soil stimulant available for scientific, educational study. Eos, Transactions American Geophysical Union, 79(34), 405-405. https://doi.org/10.1029/98eo00309</ref>=== | ||
| + | JSC Mars-1 is a simulated Martian soil developed for use in: scientific research, engineering studies, and education. | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | | | ||
| + | |Viking 1 | ||
| + | |Viking 2 | ||
| + | |Pathfinder | ||
| + | |JSC Mars-1 volatiles | ||
| + | |JSC Mars-1 dry | ||
| + | |- | ||
| + | |Oxide | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |(Wt%) | ||
| + | |- | ||
| + | |SiO<sub>2</sub> | ||
| + | |43 | ||
| + | |43 | ||
| + | |44.0 | ||
| + | |34.5 | ||
| + | |43.5 | ||
| + | |- | ||
| + | |Fe<sub>2</sub>O<sub>3</sub> | ||
| + | |18.5 | ||
| + | |17.8 | ||
| + | |16.5 | ||
| + | |12.4 | ||
| + | |15.6 | ||
| + | |- | ||
| + | |Al<sub>2</sub>O<sub>3</sub> | ||
| + | |7.3 | ||
| + | |7 | ||
| + | |7.5 | ||
| + | |18.5 | ||
| + | |23.3 | ||
| + | |- | ||
| + | |SO<sub>3</sub> | ||
| + | |6.6 | ||
| + | |8.1 | ||
| + | |4.9 | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |- | ||
| + | |MgO | ||
| + | |6 | ||
| + | |6 | ||
| + | |7.0 | ||
| + | |2.7 | ||
| + | |3.4 | ||
| + | |- | ||
| + | |CaO | ||
| + | |5.9 | ||
| + | |5.7 | ||
| + | |5.6 | ||
| + | |4.9 | ||
| + | |6.2 | ||
| + | |- | ||
| + | |Cl | ||
| + | |0.7 | ||
| + | |0.5 | ||
| + | |0.5 | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |- | ||
| + | |TiO<sub>2</sub> | ||
| + | |0.66 | ||
| + | |0.56 | ||
| + | |1.1 | ||
| + | |3.0 | ||
| + | |3.8 | ||
| + | |- | ||
| + | |MnO | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |0.2 | ||
| + | |0.3 | ||
| + | |- | ||
| + | |K<sub>2</sub>O | ||
| + | |<0.15 | ||
| + | |<0.15 | ||
| + | |0.3 | ||
| + | |0.5 | ||
| + | |0.6 | ||
| + | |- | ||
| + | |Na<sub>2</sub>O | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |2.1 | ||
| + | |1.9 | ||
| + | |2.4 | ||
| + | |- | ||
| + | |P<sub>2</sub>O<sub>5</sub> | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |0.7 | ||
| + | |0.9 | ||
| + | |- | ||
| + | |Volatiles | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |n.a. | ||
| + | |21.8 | ||
| + | |n.a. | ||
| + | |- | ||
| + | |Total | ||
| + | |89 | ||
| + | |89 | ||
| + | |89.5 | ||
| + | |101.1 | ||
| + | |100.0 | ||
| + | |} | ||
| + | |||
| + | n.a. = Not Analyzed: all iron calculated as Fe<sub>2</sub>O<sub>3</sub> | ||
| + | |||
| + | ===SNC-meteorites<ref>P. Cattermole, ''Mars: The story of the Red Planet'', (Springer Science & Business Media, Dec 6, 2012 - Science), page 51, Table 5.1.</ref>=== | ||
| + | SNC Meteorites are meteorites ejected from Mars and named SNC after the locations where they were first discovered. | ||
| + | |||
| + | {| | ||
| + | |Mantle Crust | ||
| + | |Name | ||
| + | |(%) | ||
| + | |- | ||
| + | |SiO<sub>2</sub> | ||
| + | |Silicon Dioxide | ||
| + | |44.4 | ||
| + | |- | ||
| + | |Al<sub>2</sub>O<sub>3</sub> | ||
| + | |Aluminum Oxide | ||
| + | |3.02 | ||
| + | |- | ||
| + | |FeO | ||
| + | |Iron(II) Oxide | ||
| + | |17.9 | ||
| + | |- | ||
| + | |MgO | ||
| + | |Magnesium Oxide | ||
| + | |30.2 | ||
| + | |- | ||
| + | |CaO | ||
| + | |Calcium Oxide | ||
| + | |2.45 | ||
| + | |- | ||
| + | |TiO<sub>2</sub> | ||
| + | |Titanium Dioxide | ||
| + | |0.14 | ||
| + | |- | ||
| + | |Na<sub>2</sub>O | ||
| + | |Sodium Oxide | ||
| + | |0.50 | ||
| + | |- | ||
| + | |P<sub>2</sub>O<sub>5</sub> | ||
| + | |Phosphorus Pentoxide | ||
| + | |0.16 | ||
| + | |- | ||
| + | |Cr<sub>2</sub>O<sub>3</sub> | ||
| + | |Chromium(III) Oxide | ||
| + | |0.76 | ||
| + | |- | ||
| + | |K | ||
| + | |Potassium | ||
| + | |305ppm | ||
| + | |- | ||
| + | |Ni | ||
| + | |Nickel | ||
| + | |400ppm | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[category:Mineralogy]] | ||
| + | |||
| + | ==Notes== | ||
| + | |||
| + | |||
| + | ==References== | ||
Latest revision as of 02:09, 22 September 2024
This page summarized the soil composition of Mars.
Contents
Overview
A predominant feature of the Martian surface is the iron oxide-rich dust known as regolith, giving the planet its characteristic red color. This dust is very fine and the result of years of meteorite impacts pulverizing the Martian surface spreading dust around the planet. This dust is blown globally by storms, creating massive seasonal dust storms that can last for months.
Rock formations on the surface are primarily composed of basalt - a consequence of the extensive lava flows that once existed as a result of ancient geological activity. Analysis of soil samples collected by the Viking landers in 1976 indicate iron-rich clays consistent with weathering of basaltic rocks.
There is also evidence the Martian surface may be more silica-rich than the basalt created by lava flows, similar to andesitic rocks found on Earth (rock which crystallizes from silicate minerals at intermediate temperatures).
Mars has twice as much iron oxide in its outer layers as Earth does, despite their similar origin (meteorite impacts). This is due to the geologically active (and hotter) Earth transporting much of the surface iron deep below the terrestrial surface. Mars does not have this geological advantage to produce heat, so the iron remains in the Martian regolith, giving Mars its red color.
Materials
Chemical composition of the soils on Mars is based upon the various data we have to date from Mars landers as well as SNC-meteorites believed to be from Mars. Different sites and sources contain different concentrations.
Spirit, Opportunity, Curiosity Rovers[1]
Values estimated from a graph. Measurements provided by their Alpha Particle X-ray Spectrometer (APXS). Spirit data taken from 59 samples at Gusev Crater, Opportunity data taken from 23 samples at Meridiani Planum, Curiosity data taken from a sample inside a rover wheel scuff at Gale Crater.
| Spirit | Opportunity | Curiosity | ||
| (Wt%) | (Wt%) | (Wt%) | ||
| SiO2 | Silicon Dioxide | 46.0 | 45.3 | 43.6 |
| FeO | Iron(II) Oxide | 16.0 | 18.8 | 21.3 |
| Al2O3 | Aluminum Oxide | 10.2 | 9.16 | 9.58 |
| MgO | Magnesium Oxide | 8.61 | 7.39 | 6.55 |
| CaO | Calcium Oxide | 6.27 | 6.93 | 7.39 |
| SO3 | Sulfur Trioxide | 6.13 | 5.92 | 5.16 |
| Na2O | Sodium Oxide | 3.00 | 2.20 | 2.20 |
| P2O5 | Phosphorus Pentoxide | 0.91 | 0.84 | 0.56 |
| TiO2 | Titanium Dioxide | 0.91 | 1.05 | 1.53 |
| Cl | Chlorine | 0.70 | 0.64 | 0.63 |
| K2O | Potassium Oxide | 0.42 | 0.49 | 0.63 |
| Cr2O3 | Chromium(III) Oxide | 0.35 | 0.42 | 0.42 |
| MnO | Manganese(II) Oxide | 0.28 | 0.35 | 0.42 |
| Ni | Nickel | 0.047 | 0.045 | 0.035 |
| Zn | Zinc | 0.027 | 0.033 | 0.027 |
| Br | Bromine | 0.005 | 0.008 | 0.003 |
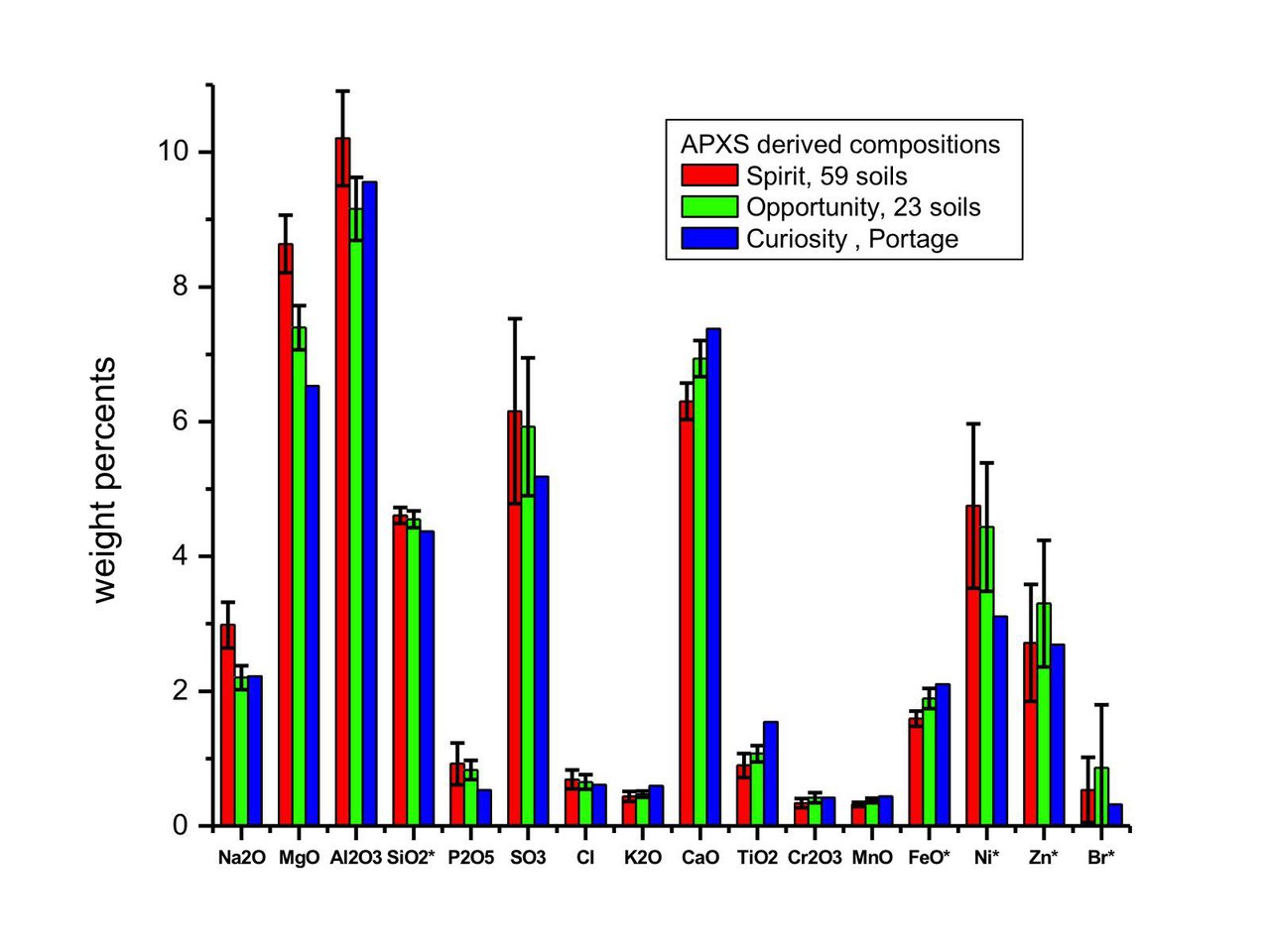 Note: In the image SiO2 and FeO values were divided by 10 to not overrun the boundaries of the image, and Ni, Zn, and Br were multiplied by 100 to be visible.
Note: In the image SiO2 and FeO values were divided by 10 to not overrun the boundaries of the image, and Ni, Zn, and Br were multiplied by 100 to be visible.
Viking Lander 1 & 2, Pathfinder, JSC Martian Soil Simulant[2]
JSC Mars-1 is a simulated Martian soil developed for use in: scientific research, engineering studies, and education.
| Viking 1 | Viking 2 | Pathfinder | JSC Mars-1 volatiles | JSC Mars-1 dry | |
| Oxide | (Wt%) | (Wt%) | (Wt%) | (Wt%) | (Wt%) |
| SiO2 | 43 | 43 | 44.0 | 34.5 | 43.5 |
| Fe2O3 | 18.5 | 17.8 | 16.5 | 12.4 | 15.6 |
| Al2O3 | 7.3 | 7 | 7.5 | 18.5 | 23.3 |
| SO3 | 6.6 | 8.1 | 4.9 | n.a. | n.a. |
| MgO | 6 | 6 | 7.0 | 2.7 | 3.4 |
| CaO | 5.9 | 5.7 | 5.6 | 4.9 | 6.2 |
| Cl | 0.7 | 0.5 | 0.5 | n.a. | n.a. |
| TiO2 | 0.66 | 0.56 | 1.1 | 3.0 | 3.8 |
| MnO | n.a. | n.a. | n.a. | 0.2 | 0.3 |
| K2O | <0.15 | <0.15 | 0.3 | 0.5 | 0.6 |
| Na2O | n.a. | n.a. | 2.1 | 1.9 | 2.4 |
| P2O5 | n.a. | n.a. | n.a. | 0.7 | 0.9 |
| Volatiles | n.a. | n.a. | n.a. | 21.8 | n.a. |
| Total | 89 | 89 | 89.5 | 101.1 | 100.0 |
n.a. = Not Analyzed: all iron calculated as Fe2O3
SNC-meteorites[3]
SNC Meteorites are meteorites ejected from Mars and named SNC after the locations where they were first discovered.
| Mantle Crust | Name | (%) |
| SiO2 | Silicon Dioxide | 44.4 |
| Al2O3 | Aluminum Oxide | 3.02 |
| FeO | Iron(II) Oxide | 17.9 |
| MgO | Magnesium Oxide | 30.2 |
| CaO | Calcium Oxide | 2.45 |
| TiO2 | Titanium Dioxide | 0.14 |
| Na2O | Sodium Oxide | 0.50 |
| P2O5 | Phosphorus Pentoxide | 0.16 |
| Cr2O3 | Chromium(III) Oxide | 0.76 |
| K | Potassium | 305ppm |
| Ni | Nickel | 400ppm |
Notes
References
- ↑ jpl.nasa.gov. 2012. Inspecting Soils Across Mars. [online] Available at: <https://www.jpl.nasa.gov/images/inspecting-soils-across-mars> [Accessed 18 October 2021].
- ↑ Allen, C., Jager, K., Morris, R., Lindstrom, D., Lindstrom, M., & Lockwood, J. (1998). Martian soil stimulant available for scientific, educational study. Eos, Transactions American Geophysical Union, 79(34), 405-405. https://doi.org/10.1029/98eo00309
- ↑ P. Cattermole, Mars: The story of the Red Planet, (Springer Science & Business Media, Dec 6, 2012 - Science), page 51, Table 5.1.







