Difference between revisions of "In-situ resource utilization"
| Line 89: | Line 89: | ||
*[[Sabatier/Water Electrolysis Process]] | *[[Sabatier/Water Electrolysis Process]] | ||
*[[Fischer-Tropsch reaction|Fischer-Tropsch Reaction]] | *[[Fischer-Tropsch reaction|Fischer-Tropsch Reaction]] | ||
| + | *[[Electrolysis]] | ||
=====Alcools===== | =====Alcools===== | ||
| Line 104: | Line 105: | ||
*Cumene process | *Cumene process | ||
| − | ===== Olefine derivatives ===== | + | =====Olefine derivatives===== |
*Wacker process | *Wacker process | ||
| Line 112: | Line 113: | ||
*Hydrogenation(Ra-Ni catalyst) | *Hydrogenation(Ra-Ni catalyst) | ||
| − | ===== Direct ethylene glycol synthesis ===== | + | =====Direct ethylene glycol synthesis===== |
| − | ====Hydrocarbon synthesis==== | + | ====[[Hydrocarbon synthesis]]==== |
| − | |||
| − | |||
[[Hydrocarbons]] can be manufactured by combining [[hydrogen]] and [[carbon]] through a variety of reactions | [[Hydrocarbons]] can be manufactured by combining [[hydrogen]] and [[carbon]] through a variety of reactions | ||
| − | ====[[Silicone Synthesis]] | + | ====[[Silicone Synthesis]] ==== |
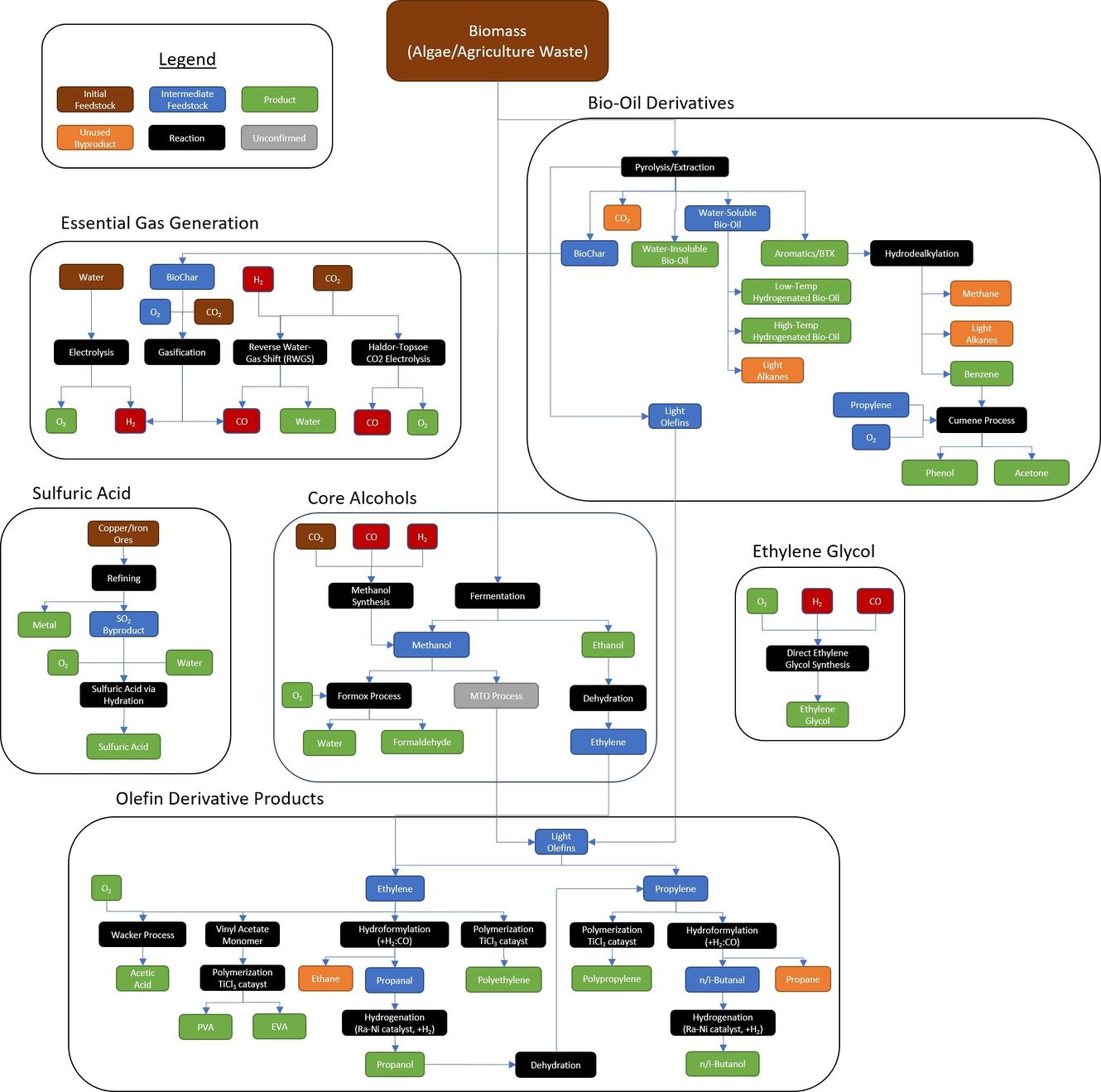
| + | Deoxidation (usually, but not exclusively) of a compound into individual elements[[File:New Essential Chemical Roadmap.jpg|thumb|1330x1330px|By Nexus Aurora, ]] | ||
| − | == | + | ==Outputs== |
| − | + | {{expandsec}} | |
| − | |||
| − | |||
| − | ==== | + | == Outputs == |
| − | |||
| − | |||
| − | |||
===Liquids=== | ===Liquids=== | ||
| Line 137: | Line 132: | ||
*Ammonia | *Ammonia | ||
*Hydrocarbons | *Hydrocarbons | ||
| + | *Ethanol | ||
| + | *Methanol | ||
| + | *Formaldehyde | ||
| + | * | ||
* | * | ||
* | * | ||
| Line 143: | Line 142: | ||
A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained from compression of the martian atmosphere, separation of excess CO2 and addition of oxygen to reach the desired proportions, that depend on the chosen atmospheric pressure in the habitats. | A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained from compression of the martian atmosphere, separation of excess CO2 and addition of oxygen to reach the desired proportions, that depend on the chosen atmospheric pressure in the habitats. | ||
| − | *Oxygen | + | *Oxygen (O2) |
| − | *Hydrogen | + | *Hydrogen (H2) |
| − | *Carbon dioxyde | + | *Carbon dioxyde (CO2) |
| − | *Carbon monoxyde | + | *Carbon monoxyde CO) |
| + | *Ethylene | ||
| + | *PRopylene | ||
===Solids=== | ===Solids=== | ||
| − | Fertilizer | + | |
| + | * Fertilizer | ||
| + | * Biochar | ||
===Habitats=== | ===Habitats=== | ||
Revision as of 15:58, 28 October 2020
The use of local resources is called in-situ resource utilization or ISRU. This concept is critical to the survival of an autonomous or semi-autonomous settlement. The ISRU timeline is very rapid for most settlement plans since the initial Zubrin proposal of MArs Direct.
Inputs
Atmosphere
Main article: Atmospheric processing
Many of the elements and molecules in the atmosphere can be utilized. Condensation, followed by distillation, are often used to extract resources. The atmosphere is first cooled to a liquid or solid state. This is distilled at precise temperatures in order to separate the elements and molecules.
- Carbon dioxide (CO2) composes 96% of the martian atmosphere. Carbon dioxide is the main source of carbon, used for fuel production (CH4) and an essential element for life. Carbon dioxide also serves as a source of oxygen for the settlement atmosphere and as the oxidizer in bi-propellant fuels.
- Nitrogen composes 2% of martian atmosphere. Nitrogen is used by plants and is part of a breathable atmosphere. Its concentration on Earth is 78% of the atmosphere.
- Argon is an inert gas, useful in some industrial processes as an inert atmosphere and may be used as propellant in Electric Propulsion of spaceships.2% of martian atmosphere
- Water (H2O) is the main source of hydrogen and oxygen. Oxygen is required for the settlement atmosphere and hydrogen is used for fuel production (CH4) and for the synthesis of hydrocarbons, the building blocks for life.
Lithosphere (surface)
Main article: Mining
Minerals in the crust of Mars must be mined and processed to be useful. The upper layer of Mars surface is called the Regolith. It is a mixtures of materials of various interest.
- Water can be gathered in a variety of ways. It is available in the form of water ice or as hydrated minerals.
- Silicates (SiO2) are useful for the production of glass and building materials. It is one of the main components of the martian planetary crust.
- Iron ore ( Hematite:Fe2O3) or (Magnetite: Fe3O4) is a source of iron and steel, as well as oxygen or CO2, depending on the process used.
- Alumina (Al2O3) is the source of aluminium. Processing also produces CO2, oxygen or water, depending on the process used.
- Calcium carbonate (CaCO3) is used for concrete production. Carbonates are also a potential source of carbon for carbohydrates. Sodium carbonates are used in glass production. Carbonates are available on Mars.[1]
- Sulfates
- Nitrates are sources of nitrogen for plants and industrial processes, ammonia and explosives. Nitrates were discovered on Mars by the Curiosity rover in 2015.
- Salts. (Mg,Na)SO4, NaCl, and (Mg,Ca)CO3. Magnesium, Calcium, Sodium, lithium, Chlorine. Practically all minerals and elements can be found in the form of salts. Sodium chloride (NaCl) is the most common salt, and is essential for life. Chlorides are likely to be abundant on Mars.[2]
- Thorium (Th) concentrations have been identified by JPL on Mars, this is the preferred fuel in a number of Molten Salt Reactor designs. [3]
Energy
Main article: Energy
Energy is required to carry out ISRU. There are three known sources of energy on Mars: the Sun, Mars' crust and nuclear fission. Energy may be stored in a variety of ways for when the sources are not available.
- Solar energy is a very variable energy source on Mars, unless some form Space Solar energy is used. It requires some form of energy storage in the settlement or supplementary energy sources, most likely nuclear.
- Nuclear energy is a stable energy source and could be used extensively on Mars. The availability of nuclear fuel on Mars needs to be explored and usable ore deposits found for a self sustainable settlement.
- Geothermal energy may be available in some martian sites.
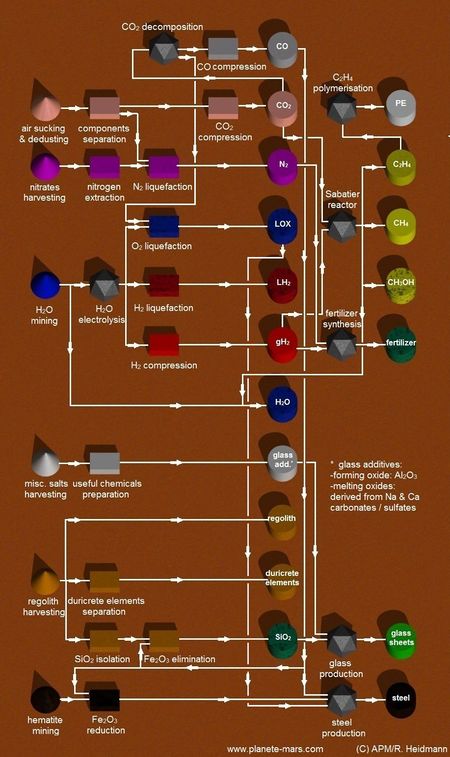
Processes
Inputs need to be processed to create outputs
Mechanical processes
- Mechanical compression of gases increases their density
- Heating and cooling are important processes that can be used to accomplish phase changes in various substances.
- Crushing, milling. These are mechanical processes that break minerals down to individual crystals for separation and materials handling. Complex minerals such as basalts, granites or ores can be broken down for separation
- Separation Mechanical, centrifugal,
- Flotation,
- Distillation,
- Condensation
Chemical processes
Gases
- Methanol synthesis
- Gasification
- Reverse Water-Gas Shift Reaction
- Haldor Topsoe CO2 electrolysis
- Sabatier/Water Electrolysis Process
- Fischer-Tropsch Reaction
- Electrolysis
Alcools
- Methanol synthesis
- Fermentation
- Formox process
- MTO process
- Dehydration
Bio-oil derivatives
- Pyrolysis/extraction
- Hydrodealkysation
- Cumene process
Olefine derivatives
- Wacker process
- Vinyl acetate monomer
- Polymerization TiCl3 catalyst
- Hydroformylation
- Hydrogenation(Ra-Ni catalyst)
Direct ethylene glycol synthesis
Hydrocarbon synthesis
Hydrocarbons can be manufactured by combining hydrogen and carbon through a variety of reactions
Silicone Synthesis
Deoxidation (usually, but not exclusively) of a compound into individual elements
Outputs
| This section of the article is incomplete or needs more detail. You can help Marspedia by expanding or correcting it. |
Outputs
Liquids
- Water. is essential for life. It is also a common process reagent, an excellent coolant for industrial processes and a source of hydrogen and oxygen using electrolysis. On Mars it can also be used as a construction material or as radiation shielding. It can be condensed out of the atmosphere or extracted from the regolith.
- Ammonia
- Hydrocarbons
- Ethanol
- Methanol
- Formaldehyde
Gases
A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained from compression of the martian atmosphere, separation of excess CO2 and addition of oxygen to reach the desired proportions, that depend on the chosen atmospheric pressure in the habitats.
- Oxygen (O2)
- Hydrogen (H2)
- Carbon dioxyde (CO2)
- Carbon monoxyde CO)
- Ethylene
- PRopylene
Solids
- Fertilizer
- Biochar
Habitats
Habitats, including living and production areas, are assembled from manufactured products or possible naturally occurring areas sur as lava tubes, to create living areas for the colonists, plants and animals.
Food production
Agriculture
Plants are natural factories, capable of utilizing the atmosphere and regolith to grow and reproduce.
Manufactured Products
Propellant
Propellant is one of the main ISRU products. It is required to make transportation less prohibitively expensive.
Cements, concretes and compressed regolith
Iron and steel
Iron and steel
Aluminium
Glass
Glass is one of the most common building materials on Earth and should be common on Mars as well, since it has unique properties of low cost and transparency. Silica, the main component of glass, is also the most common material in the martian crust.