Difference between revisions of "Carbon Dioxide Scrubbers"
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Stefan}} | {{Stefan}} | ||
| − | A steady supply of oxygen alone is insufficient to keep astronauts breathing. While the intake of oxygen is essential for respiration, the by-product of this respiration is carbon dioxide (CO<sub>2</sub>), a toxic gas which, depending on the | + | [[File:Apollo13Scrubber.jpg|thumb|right|The crew of Apollo 13 famously improvised an adapter for their CO<sub>2</sub> scrubber in order to avoid a deadly build-up of the toxic gas.]]A steady supply of [[oxygen]] alone is insufficient to keep astronauts breathing. While the intake of oxygen is essential for respiration, the by-product of this respiration is [[carbon dioxide]] (CO<sub>2</sub>), a toxic gas which, depending on the extent of its concentration, can cause symptoms varying from mild discomfort to death. In the sealed environment of a spacecraft or a [[Habitat|Mars habitat]], this gas must be carefully monitored and removed from the air in a process called CO<sub>2</sub> scrubbing. Spacecraft have until now used a variety of artificial means to accomplish this, although plant-based air recycling is a theoretical possibility which has been tested in some experimental settings. |
==Dangers of Excessive CO<sub>2</sub> Concentration== | ==Dangers of Excessive CO<sub>2</sub> Concentration== | ||
| − | A human being exhales approximately one kilogram of CO<sub>2</sub> per day.<ref name=":1">James, J. T., & Macatangay, A. (2009). ''Carbon Dioxide – Our Common “Enemy.”'' 8.</ref> The concentration of this gas aboard the International Space Station averages about 0.4%, ten times that of Earth's atmosphere.<ref name=":8" /> At these levels, side effects vary from person to person, with some astronauts unaffected and others experiencing headaches and irritability. At higher levels, however, CO<sub>2</sub> can quickly become toxic: | + | A human being exhales approximately one kilogram of CO<sub>2</sub> per day.<ref name=":1">James, J. T., & Macatangay, A. (2009). ''Carbon Dioxide – Our Common “Enemy.”'' 8.</ref> The concentration of this gas aboard the [[International Space Station]] averages about 0.4%, ten times that of Earth's atmosphere.<ref name=":8" /> At these levels, side effects vary from person to person, with some astronauts unaffected and others experiencing headaches and irritability. At higher levels, however, CO<sub>2</sub> can quickly become toxic: |
| − | {| class="wikitable" | + | {| class="wikitable" |
|+Symptoms Experienced According to CO<sub>2</sub> Level<ref name=":0">Freudenrich, C. (2011). How is carbon dioxide eliminated aboard a spacecraft? | HowStuffWorks. Retrieved August 15, 2019, from HowStuffWorks website: <nowiki>https://science.howstuffworks.com/carbon-dioxide-eliminated-aboard-spacecraft.htm</nowiki></ref> | |+Symptoms Experienced According to CO<sub>2</sub> Level<ref name=":0">Freudenrich, C. (2011). How is carbon dioxide eliminated aboard a spacecraft? | HowStuffWorks. Retrieved August 15, 2019, from HowStuffWorks website: <nowiki>https://science.howstuffworks.com/carbon-dioxide-eliminated-aboard-spacecraft.htm</nowiki></ref> | ||
!CO<sub>2</sub> Concentration | !CO<sub>2</sub> Concentration | ||
| Line 24: | Line 24: | ||
|Death | |Death | ||
|} | |} | ||
| − | NASA has set strict limits of acceptable CO<sub>2</sub> | + | [[National Aeronautics and Space Administration (NASA)|NASA]] has set strict limits of acceptable CO<sub>2</sub> concentrations on spacecraft. The longer the duration of the flight, the lower the permissible maximum: for example, while allowing a maximum of 2% for a one-hour period, NASA recommends that the concentrations not exceed 0.5% over a 1000-day stay<ref name=":1" /> (an as-yet hypothetical duration, as the record for longest consecutive stay in space at the time of writing is held by Russian cosmonaut Valery Polyakov at 438 days<ref>Wall, M. (2019). Most Extreme Human Spaceflight Records of All Time | Space. Retrieved August 15, 2019, from Soace.com website: <nowiki>https://www.space.com/11337-human-spaceflight-records-50th-anniversary.html</nowiki></ref>). The proportionate decrease in concentration acceptability in accordance with duration accounts for the fact that the longer the period of time, the higher the likelihood of CO<sub>2</sub>-induced behavioral changes negatively affecting missions requiring close personal contact in confined spaces. |
| − | Moderating CO<sub>2</sub> levels over such a long period of time naturally implies weight and power demands, yet NASA has adopted more stringent regulations than the Navy, which allows up to 2.5% concentrations for submarine personnel over a 24-hour period.<ref name=":1" /> While both types of crews operate in a confined area with an artificially-regulated atmosphere, repairing a CO<sub>2</sub> removal system in space constitutes a potentially more complex task where one cannot simply surface in the event of an unsuccessful attempt brought on by concentration difficulties. Hypothetically, a well-developed Mars colony could relax such limitations slightly once it is large enough to contain multiple fail-safes and back-ups, thereby reducing the energy demands on the colony as a whole. | + | Moderating CO<sub>2</sub> levels over such a long period of time naturally implies weight and power demands, yet NASA has adopted more stringent regulations than the Navy, which allows up to 2.5% concentrations for submarine personnel over a 24-hour period.<ref name=":1" /> While both types of crews operate in a confined area with an artificially-regulated atmosphere, repairing a CO<sub>2</sub> removal system in space constitutes a potentially more complex task where one cannot simply surface in the event of an unsuccessful attempt brought on by concentration difficulties. Hypothetically, a well-developed [[Colony|Mars colony]] could relax such limitations slightly once it is large enough to contain multiple fail-safes and back-ups, thereby reducing the energy demands on the colony as a whole. Even so, the discomfort caused by extended exposure to high CO<sub>2</sub> concentrations means that maintaining them at near-Earth levels will likely remain a high priority. |
| − | In the days of interplanetary travel before colonists establish a self-sustaining colony, missions could screen crews for CO<sub>2</sub>-resistant individuals.<ref name=":8" /> As mentioned above, the effects of elevated CO<sub>2</sub> concentrations aboard the ISS have affected astronauts to differing degrees—because higher permissible levels of CO<sub>2</sub> mean less demand on scrubbing systems, ensuring crews consist only of members whose performance remains unchanged in the presence of increased concentrations could provide | + | In the days of interplanetary travel before colonists establish a self-sustaining colony, missions could also simply screen crews for CO<sub>2</sub>-resistant individuals.<ref name=":8" /> As mentioned above, the effects of elevated CO<sub>2</sub> concentrations aboard the ISS have affected astronauts to differing degrees—because higher permissible levels of CO<sub>2</sub> mean less demand on scrubbing systems, ensuring crews consist only of members whose performance remains unchanged in the presence of increased concentrations could provide a straightforward way to reduce mission overhead. |
==Artificial CO<sub>2</sub> Scrubbers== | ==Artificial CO<sub>2</sub> Scrubbers== | ||
| Line 34: | Line 34: | ||
===Chemical Systems=== | ===Chemical Systems=== | ||
| − | These systems scrub CO<sub>2</sub> from the air by bringing it into contact with a substance which attaches itself to the CO<sub>2</sub> in a chemical reaction. Scrubbers can accomplish this with soda lime (a mixture of chemicals including calcium hydroxide, sodium hydroxide, and potassium hydroxide), amines (a derivative of ammonia), or potassium superoxide (a substance used in firefighter and mining rebreathers), but the low molecular weight and corresponding transport costs of lithium hydroxide (LiOH) has led to its popularity aboard spacecraft.<ref name=":0" /><ref name=":5" /> | + | These systems scrub CO<sub>2</sub> from the air by bringing it into contact with a substance which attaches itself to the CO<sub>2</sub> in a chemical reaction. Scrubbers can accomplish this with soda lime (a mixture of chemicals including calcium hydroxide, sodium hydroxide, and potassium hydroxide), amines (a derivative of ammonia), or potassium superoxide (a substance used in firefighter and mining rebreathers), but the low molecular weight and corresponding transport costs of lithium hydroxide (LiOH) has led to its popularity aboard spacecraft.<ref name=":0" /><ref name=":5" /> Bringing CO<sub>2</sub> into contact with LiOH creates lithium carbonate (Li<sub>2</sub>CO<sub>3)</sub> and [[water]] through the following reaction: |
CO<sub>2</sub> (g) + 2LiOH (s) → Li<sub>2</sub>CO<sub>3</sub> (s) + H<sub>2</sub>O (l) | CO<sub>2</sub> (g) + 2LiOH (s) → Li<sub>2</sub>CO<sub>3</sub> (s) + H<sub>2</sub>O (l) | ||
| − | [[File:LiOH_Cannister.png|left|thumb|Lithium Hydroxide canister used aboard the space shuttle with 16 cm ruler for scale. Air is blown through the central annulus, where it moves through a perforated inner layer to come into contact with the LiOH. The remaining, scrubbed | + | [[File:LiOH_Cannister.png|left|thumb|Lithium Hydroxide canister used aboard the [[Space Shuttle to Mars|space shuttle]] with 16 cm ruler for scale. Air is blown through the central annulus, where it moves through a perforated inner layer to come into contact with the LiOH. The remaining air, now scrubbed of CO<sub>2</sub>, exhausts through the outer perimeter and is recycled back into the spacecraft.<ref name=":6">Matty, C. (2010). Overview of Carbon Dioxide Control Issues During International Space Station/Space Shuttle Joint Docked Operations. ''40th International Conference on Environmental Systems''. Presented at the 40th International Conference on Environmental Systems, Barcelona, Spain. <nowiki>https://doi.org/10.2514/6.2010-6251</nowiki></ref>]]The conversion to Li<sub>2</sub>CO<sub>3</sub> is irreversible, meaning that the canisters used for the reaction must be replaced once all of the LiOH is consumed.<ref name=":6" /> |
| − | Apollo 13 provides perhaps the most famous instance of complications arising due to this replacement process:<ref name=":0" /> an explosion in the command module forced all three | + | Apollo 13 provides perhaps the most famous instance of complications arising due to this replacement process:<ref name=":0" /> an explosion in the command module forced all three crew members into the lunar module, whose air recycling system was designed for only two astronauts. The lunar module's LiOH canisters quickly became saturated, and the crew had to improvise extensive modifications using hoses, socks, plastic bags, and duct tape in order to adapt the command module's square canisters to the round lunar module system. |
In addition to the Apollo program, LiOH canisters constituted the primary CO<sub>2</sub> removal system during Mercury, Gemini, and Space Shuttle missions.<ref name=":1" /> One canister acted as the primary scrubber, with another in place as a backup; as the primary canister became depleted, the backup took over and the primary was replaced, with this alternating cycle repeating as many times as necessary until the crew returned to Earth. The longer the mission, the more canisters required. | In addition to the Apollo program, LiOH canisters constituted the primary CO<sub>2</sub> removal system during Mercury, Gemini, and Space Shuttle missions.<ref name=":1" /> One canister acted as the primary scrubber, with another in place as a backup; as the primary canister became depleted, the backup took over and the primary was replaced, with this alternating cycle repeating as many times as necessary until the crew returned to Earth. The longer the mission, the more canisters required. | ||
| − | Scrubbing the CO<sub>2</sub> which a single crew member exhales over the course of a day requires roughly 1.5 kilograms of LiOH.<ref name=":1" /> Modern LiOH canisters used by NASA contain 3.0 kilograms of LiOH pellets, and the water created during the chemical reaction brings the weight of a depleted canister to around 4.0 kilograms.<ref name=":1" /><ref name=":6" /> Russian Soyuz canisters are almost twice as large. This weight, along with the size of the canisters (approximately 6 liters in volume), adds substantial considerations not only to the cost of launching from Earth, but also to returning, since the canisters are not jettisoned into space.<ref name=":1" /> | + | Scrubbing the CO<sub>2</sub> which a single crew member exhales over the course of a day requires roughly 1.5 kilograms of LiOH.<ref name=":1" /> Modern LiOH canisters used by NASA contain 3.0 kilograms of LiOH pellets, and the water created during the chemical reaction brings the weight of a depleted canister to around 4.0 kilograms.<ref name=":1" /><ref name=":6" /> Russian [[Soyuz-Fregat rocket|Soyuz]] canisters are almost twice as large. This weight, along with the size of the canisters (approximately 6 liters in volume), adds substantial considerations not only to the cost of launching from Earth, but also to returning, since the canisters are not jettisoned into space.<ref name=":1" /> |
| − | Because the number of required canisters increases in direct proportion to mission length, regenerative CO<sub>2</sub> scrubbers become more cost effective than a system based on consumable LiOH after a period of 20-30 days.<ref name=":7" /> Skylab and Mir still employed LiOH canisters in a supplementary capacity, as does the International Space Station, which maintains a minimum contingency reserve capable of sustaining 3 crew members for 15 days, as well as a stockpile from which docking craft can draw when payload requirements restrict the amount available for their own scrubbing needs.<ref name=":1" /> The primary system employed on the ISS, however, utilizes regenerative filtering technology. | + | Because the number of required canisters increases in direct proportion to mission length, regenerative CO<sub>2</sub> scrubbers become more cost effective than a system based on consumable LiOH after a period of 20-30 days.<ref name=":7">Closing the Loop: Recycling Water and Air in Space. (n.d.). Retrieved August 15, 2019, from NASA.gov website: <nowiki>https://www.nasa.gov/pdf/146558main_RecyclingEDA(final)%204_10_06.pdf</nowiki></ref> Skylab and Mir still employed LiOH canisters in a supplementary capacity, as does the International Space Station, which maintains a minimum contingency reserve capable of sustaining 3 crew members for 15 days, as well as a stockpile from which docking craft can draw when payload requirements restrict the amount available for their own scrubbing needs.<ref name=":1" /> The primary system employed on the ISS, however, utilizes regenerative filtering technology. |
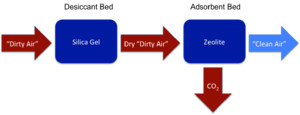
===Adsorption Systems=== | ===Adsorption Systems=== | ||
| Line 56: | Line 56: | ||
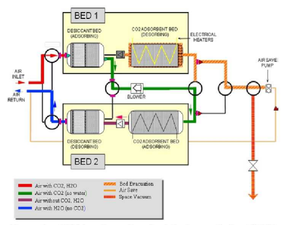
[[File:CDRAArchitecture.png|thumb|right|Diagram showing the complete path of air through the alternating adsorption/desorption system of the CDRA.]]The CDRA system utilizes two sets of these two-bed combinations in alternating conjunction with one another, with each set containing one desiccant bed and one CO<sub>2</sub> adsorption bed. At any given time, one of these two beds in a set is adsorbing while the other is desorbing, and vice versa for the other set. That is, if the desiccant bed of one set is currently adsorbing water, then the CO<sub>2</sub> bed in the same set as well as the desiccant bed of the opposite set will be desorbing.<ref name=":6" /> In this way, half of the system is always removing water and CO<sub>2</sub> while the other half is regenerating its capability to do so. | [[File:CDRAArchitecture.png|thumb|right|Diagram showing the complete path of air through the alternating adsorption/desorption system of the CDRA.]]The CDRA system utilizes two sets of these two-bed combinations in alternating conjunction with one another, with each set containing one desiccant bed and one CO<sub>2</sub> adsorption bed. At any given time, one of these two beds in a set is adsorbing while the other is desorbing, and vice versa for the other set. That is, if the desiccant bed of one set is currently adsorbing water, then the CO<sub>2</sub> bed in the same set as well as the desiccant bed of the opposite set will be desorbing.<ref name=":6" /> In this way, half of the system is always removing water and CO<sub>2</sub> while the other half is regenerating its capability to do so. | ||
| − | Air first passes over a desaturated desiccant bed, removing moisture which can inhibit the function of the adsorbing CO<sub>2</sub> bed in the next step. After the CO<sub>2</sub> is removed, the dry, scrubbed air passes over a saturated desiccant bed, which re-humidifies it before returning it to the cabin. Once the CO<sub>2</sub> bed is fully saturated, it can then be vented directly into space or combined with hydrogen gas to form water and methane gas using the onboard Sabatier system. When the previously adsorbing beds become saturated, air is pumped the opposite way through the system while the saturated beds desorb and the now-desaturated beds begin to adsorb again. | + | Air first passes over a desaturated desiccant bed, removing moisture which can inhibit the function of the adsorbing CO<sub>2</sub> bed in the next step. After the CO<sub>2</sub> is removed, the dry, scrubbed air passes over a saturated desiccant bed, which re-humidifies it before returning it to the cabin. Once the CO<sub>2</sub> bed is fully saturated, it can then be vented directly into space or combined with hydrogen gas to form water and [[methane]] gas using the onboard [[Sabatier/Water Electrolysis Process|Sabatier]] system. When the previously adsorbing beds become saturated, air is pumped the opposite way through the system while the saturated beds desorb and the now-desaturated beds begin to adsorb again. |
| − | In theory, this process can continue indefinitely and therefore provides an attractive alternative to chemical scrubbers. Even so, CDRA has suffered its share of complications.<ref name=":6" /> The zeolite of the desiccant and CO<sub>2</sub> adsorption beds takes the form of loose pellets, and because the process necessitates blowing air over these beds, early implementations experienced problems with | + | In theory, this process can continue indefinitely and therefore provides an attractive alternative to chemical scrubbers. Even so, CDRA has suffered its share of complications.<ref name=":6" /> The zeolite of the desiccant and CO<sub>2</sub> adsorption beds takes the form of loose pellets, and because the process necessitates blowing air over these beds, early implementations experienced problems with the pellets being blown off the beds and into air valves. Zeolite dust has also accumulated to the point where airflow became restricted beyond functional levels, and electrical shorts have caused additional mechanical problems. These issues have been addressed through various design revisions,<ref name=":1" /><ref name=":6" /> but demonstrate the drawbacks inherent in a complicated system such as CDRA, despite the advantages of its regenerative nature. |
===Amine-based systems=== | ===Amine-based systems=== | ||
| Line 74: | Line 74: | ||
*[[Carbon monoxide#High temperature electrolysis|Solid oxide electrolysis]] to produce carbon monoxide and oxygen. | *[[Carbon monoxide#High temperature electrolysis|Solid oxide electrolysis]] to produce carbon monoxide and oxygen. | ||
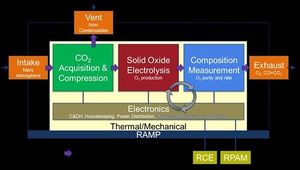
| − | [[File:MOXIEFlowChart.jpg|thumb|left|MOXIE will produce oxygen from CO<sub>2</sub>, check it for purity, and then release it back into the atmosphere.]]The last of these processes will be tested on Mars aboard the Mars 2020 MOXIE (Mars Oxygen ISRU Experiment) project.<ref name=":9">MOXIE for Scientists. (n.d.). Retrieved August 18, 2019, from Mars.Nasa.gov website: <nowiki>https://mars.nasa.gov/mars2020/mission/instruments/moxie/for-scientists/</nowiki></ref> In contrast to spacecraft, where CO<sub>2</sub> scrubbers seek to isolate quantities of the gas which are relatively small in comparison to the overall air content, Mars has an atmosphere almost entirely (96%) composed of CO<sub>2</sub>. This means that with the help of ISRU techniques, a vast supply of oxygen for both breathing and rocket fuel can be obtained without shipping it from Earth. Should the MOXIE prototype prove successful, scaled-up versions 100 times its size could save billions of dollars per launch.<ref name=":10">Orwig, J. (2015). Scientists have a plan to make breathable oxygen on Mars for the first time. Retrieved August 18, 2019, from Business Insider website: <nowiki>https://www.businessinsider.com/moxie-makes-oxygen-for-colonies-on-mars-2015-3</nowiki></ref> | + | [[File:MOXIEFlowChart.jpg|thumb|left|MOXIE will produce oxygen from CO<sub>2</sub>, check it for purity, and then release it back into the atmosphere.]]The last of these processes will be tested on Mars aboard the [[Mars 2020]] MOXIE (Mars Oxygen ISRU Experiment) project.<ref name=":9">MOXIE for Scientists. (n.d.). Retrieved August 18, 2019, from Mars.Nasa.gov website: <nowiki>https://mars.nasa.gov/mars2020/mission/instruments/moxie/for-scientists/</nowiki></ref> In contrast to spacecraft, where CO<sub>2</sub> scrubbers seek to isolate quantities of the gas which are relatively small in comparison to the overall air content, Mars has an [[Atmosphere|atmosphere almost entirely (96%) composed of CO<sub>2</sub>]]. This means that with the help of [[List of ISRU|ISRU techniques]], a vast supply of oxygen for both breathing and rocket fuel can be obtained without shipping it from Earth. Should the MOXIE prototype prove successful, scaled-up versions 100 times its size could save billions of dollars per launch.<ref name=":10">Orwig, J. (2015). Scientists have a plan to make breathable oxygen on Mars for the first time. Retrieved August 18, 2019, from Business Insider website: <nowiki>https://www.businessinsider.com/moxie-makes-oxygen-for-colonies-on-mars-2015-3</nowiki></ref> |
| − | MOXIE will collect atmospheric CO<sub>2</sub>, pressurize it, and then electrochemically split it in a process similar to running a fuel cell in reverse.<ref name=":9" /> The intention of the experiment at this stage is not to build up any stockpile of oxygen on the planet, but merely to serve as a proof of concept for future missions—all products of the reaction will be released back into the atmosphere after the oxygen is checked for purity and the gases are filtered | + | MOXIE will collect atmospheric CO<sub>2</sub>, pressurize it, and then electrochemically split it in a process similar to running a [[fuel cell]] in reverse.<ref name=":9" /> The intention of the experiment at this stage is not to build up any stockpile of oxygen on the planet, but merely to serve as a proof of concept for future missions—all products of the reaction will be released back into the atmosphere after the oxygen is checked for purity and the gases are filtered in accordance with planetary protection requirements.<ref name=":10" /> The thermal requirements of the system are extensive, but it has the advantage of being able to produce an unlimited amount of oxygen without the addition of any other off-planet substances.<ref>Zubrin, R. (2011). ''The Case for Mars''. Simon & Schuster. Page 165.</ref> |
==Biological CO<sub>2</sub> Scrubbers== | ==Biological CO<sub>2</sub> Scrubbers== | ||
| − | The chief advantage of a biological recycling system over a mechanically-sustained one is simple: plants don't break. Where a machine might suddenly cease to function and require expensive parts shipped from Earth, nature has largely optimized self-repair via evolution. Given the relatively simple level of technology involved in proper lighting, plumbing, and pumps, and provided that no pests or insects are introduced to the sterile conditions of space, plants could potentially be far more reliable than artificial CO<sub>2</sub> scrubbers.<ref name=":4" /> Furthermore, the right plants could also double as a food source for astronauts or colonists. | + | The chief advantage of a biological recycling system over a mechanically-sustained one is simple: [[plants]] don't break. Where a machine might suddenly cease to function and require expensive parts shipped from Earth, nature has largely optimized self-repair via evolution. Given the relatively simple level of technology involved in proper [[lighting]], [[Settlement systems#Plumbing and Water distribution|plumbing]], and pumps, and provided that [[How living on Mars will be different than living on Earth#World without pests|no pests or insects are introduced to the sterile conditions of space]], plants could potentially be far more reliable than artificial CO<sub>2</sub> scrubbers.<ref name=":4" /> Furthermore, the right plants could also double as a [[Food#Food and crop energy and yields|food source]] for astronauts or colonists. |
===Previous Experiments=== | ===Previous Experiments=== | ||
| − | Several closed-ecosystem experiments conducted on Earth have investigated the viability of a plant-sustained air recycling system. In the 1970s, the BIOS-3 facility in Siberia found that 8m<sup>2</sup> of Chlorella algae could maintain the 0<sub>2</sub>/CO<sub>2</sub> balance for one individual in a 315 m<sup>3</sup> environment.<ref name=":2">Villazon, L. (n.d.). How many plants would I need in an airtight room to be able to breathe? Retrieved August 16, 2019, from BBC Science Focus Magazine website: <nowiki>https://www.sciencefocus.com/science/how-many-plants-would-i-need-in-an-airtight-room-to-be-able-to-breathe/</nowiki></ref> [[File:Biosphere2.jpg|left|thumb|The Biosphere 2 facility tested the potential of a closed, biologically self-sustained ecosystem]]The Biosphere 2 project in Arizona attempted a larger-scale project over the course of 2 years, sealing 8 participants in a 180,000 m<sup>3</sup> facility with an artificial ecosystem.<ref name=":3">Nelson, M., Dempster, W., Alvarez-Romo, N., & MacCallum, T. (1994). Atmospheric dynamics and bioregenerative technologies in a soil-based ecological life support system: Initial results from biosphere 2. ''Advances in Space Research'', ''14''(11), 417–426. <nowiki>https://doi.org/10.1016/0273-1177(94)90331-X</nowiki></ref> The facility operated in mostly closed-loop conditions, but was exposed to natural sunlight. Daily variance in this light caused CO<sub>2</sub> levels to fluctuate substantially according to the relative activity of photosynthesis: while CO<sub>2</sub> concentrations dropped during the day, they rose sharply at night when respiration predominated. Seasonal variation was similarly evident, with levels rising in low-light winter months and on cloudy days, while longer sun exposures corresponding with summer months resulted in lower concentrations. December, for example, averaged CO<sub>2</sub> levels over twice as high as those in June, with the two months differing by up to 5 hours of sun exposure at their extreme ends. | + | Several closed-ecosystem experiments conducted on Earth have investigated the viability of a plant-sustained air recycling system. In the 1970s, the BIOS-3 facility in Siberia found that 8m<sup>2</sup> of Chlorella [[algae]] could maintain the 0<sub>2</sub>/CO<sub>2</sub> balance for one individual in a 315 m<sup>3</sup> environment.<ref name=":2">Villazon, L. (n.d.). How many plants would I need in an airtight room to be able to breathe? Retrieved August 16, 2019, from BBC Science Focus Magazine website: <nowiki>https://www.sciencefocus.com/science/how-many-plants-would-i-need-in-an-airtight-room-to-be-able-to-breathe/</nowiki></ref> [[File:Biosphere2.jpg|left|thumb|The Biosphere 2 facility tested the potential of a closed, biologically self-sustained ecosystem]]The Biosphere 2 project in Arizona attempted a larger-scale project over the course of 2 years, sealing 8 participants in a 180,000 m<sup>3</sup> facility with an artificial ecosystem.<ref name=":3">Nelson, M., Dempster, W., Alvarez-Romo, N., & MacCallum, T. (1994). Atmospheric dynamics and bioregenerative technologies in a soil-based ecological life support system: Initial results from biosphere 2. ''Advances in Space Research'', ''14''(11), 417–426. <nowiki>https://doi.org/10.1016/0273-1177(94)90331-X</nowiki></ref> The facility operated in mostly closed-loop conditions, but was exposed to natural [[sunlight]]. Daily variance in this light caused CO<sub>2</sub> levels to fluctuate substantially according to the relative activity of photosynthesis: while CO<sub>2</sub> concentrations dropped during the day, they rose sharply at night when respiration predominated. Seasonal variation was similarly evident, with levels rising in low-light winter months and on cloudy days, while longer sun exposures corresponding with summer months resulted in lower concentrations. December, for example, averaged CO<sub>2</sub> levels over twice as high as those in June, with the two months differing by up to 5 hours of sun exposure at their extreme ends. |
| − | The project utilized various methods to attenuate the effects of this variance, including CO<sub>2</sub> sequestration via calcium carbonate precipitation, lowering nighttime temperatures to reduce respiration, and storing trimmed biomass in dry areas to slow its decomposition. Notably, an unanticipated, steady decline in oxygen levels—which required the artificial injection of fresh oxygen after dropping to a point where team members were suffering symptoms of altitude sickness—was attributed to additional microbial respiration from organically-enriched soil.<ref>Nelson, M. (2018). Biosphere 2: What Really Happened? Retrieved August 16, 2019, from Dartmouth Alumni Magazine website: <nowiki>https://dartmouthalumnimagazine.com/articles/biosphere-2-what-really-happened</nowiki></ref> Unsealed concrete in the habitat absorbed the CO<sub>2</sub> released by this respiration, and therefore prevented its re-conversion into oxygen via photosynthesis. | + | The project utilized various methods to attenuate the effects of this variance, including CO<sub>2</sub> sequestration via calcium carbonate precipitation, lowering nighttime temperatures to reduce respiration, and storing trimmed [[biomass]] in dry areas to slow its decomposition. Notably, an unanticipated, steady decline in oxygen levels—which required the artificial injection of fresh oxygen after dropping to a point where team members were suffering symptoms of altitude sickness—was attributed to additional [[Microbes|microbial]] respiration from organically-enriched [[soil]].<ref>Nelson, M. (2018). Biosphere 2: What Really Happened? Retrieved August 16, 2019, from Dartmouth Alumni Magazine website: <nowiki>https://dartmouthalumnimagazine.com/articles/biosphere-2-what-really-happened</nowiki></ref> Unsealed [[concrete]] in the habitat absorbed the CO<sub>2</sub> released by this respiration, and therefore prevented its re-conversion into oxygen via photosynthesis. |
===Potential for Future Missions=== | ===Potential for Future Missions=== | ||
| − | With further investigation, these plant-based systems could potentially | + | With further investigation, these plant-based systems could potentially fulfill the air-recycling needs of future space expeditions. Biological means have yet to feature as the primary form of CO<sub>2</sub> scrubbing for spacecraft due to a variety of reasons including mass constraints, the number of plants required per individual, and the energy costs of optimized lighting and air conditioning.<ref name=":7" /><ref name=":3" /> The International Space Station, for example, has plants aboard, but not in the quantities necessary to have any appreciable impact on CO<sub>2</sub> concentrations.<ref name=":8">Dunn, T. (2015). Dissecting the Technology of “The Martian”: Air—Tested. Retrieved August 15, 2019, from Tested.com website: <nowiki>https://www.tested.com/science/538792-dissecting-technology-martian-air/</nowiki></ref> |
Yet, the above experiments hint at the possibility of an exclusively bioregenerative system. The ISS has a habitable volume (388 m<sup>3</sup>) slightly larger than the 315 m<sup>3</sup> environment of the successful BIOS-3 experiment, and a pressurized volume on the order of 3 times as large at 932 m<sup>3</sup>.<ref>Garcia, M. (2019). International Space Station Facts and Figures [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/feature/facts-and-figures</nowiki></ref> The BIOS-3 experiments supported up to 3 individuals for 180 days,<ref name=":2" /> meaning that the ISS could theoretically recycle air for a crew of at least 8.<ref name=":4">Walker, R. (2015). Could Astronauts Get All Their Oxygen From Algae Or Plants? And Their Food Also? | Science 2.0. Retrieved August 16, 2019, from Science20.com website: <nowiki>https://www.science20.com/robert_inventor/could_astronauts_get_all_their_oxygen_from_algae_or_plants_and_their_food_also-156990</nowiki></ref> | Yet, the above experiments hint at the possibility of an exclusively bioregenerative system. The ISS has a habitable volume (388 m<sup>3</sup>) slightly larger than the 315 m<sup>3</sup> environment of the successful BIOS-3 experiment, and a pressurized volume on the order of 3 times as large at 932 m<sup>3</sup>.<ref>Garcia, M. (2019). International Space Station Facts and Figures [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/feature/facts-and-figures</nowiki></ref> The BIOS-3 experiments supported up to 3 individuals for 180 days,<ref name=":2" /> meaning that the ISS could theoretically recycle air for a crew of at least 8.<ref name=":4">Walker, R. (2015). Could Astronauts Get All Their Oxygen From Algae Or Plants? And Their Food Also? | Science 2.0. Retrieved August 16, 2019, from Science20.com website: <nowiki>https://www.science20.com/robert_inventor/could_astronauts_get_all_their_oxygen_from_algae_or_plants_and_their_food_also-156990</nowiki></ref> | ||
| − | The algae in these experiments used 18 kW of artificial lighting per person; extrapolating this to 108 kW for a crew of six on the ISS—which could not rely on natural light alone due to regularly being in darkness—would consume the majority of the 120 kW generated by the station's solar arrays.<ref name=":4" /><ref>Garcia, M. (2017, July 31). About the Space Station Solar Arrays [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/mission_pages/station/structure/elements/solar_arrays-about.html</nowiki></ref> The Russian lamps, however, used xenon technology,<ref name=":4" /> which is anywhere from ½ to 10 times less efficient than modern LEDs in terms of lumens generated per watt.<ref>Luminous efficacy. (2019). In ''Wikipedia''. Retrieved from <nowiki>https://en.wikipedia.org/w/index.php?title=Luminous_efficacy&oldid=903091057</nowiki></ref> More efficient lighting could therefore theoretically reduce the energy requirements to somewhere between 11-54 kW, a much more feasible range considering the power output of the ISS. | + | The algae in these experiments used 18 kW of artificial lighting per person; extrapolating this to 108 kW for a crew of six on the ISS—which could not rely on natural light alone due to regularly being in darkness—would consume the majority of the 120 kW generated by the station's [[Solar panel|solar arrays]].<ref name=":4" /><ref>Garcia, M. (2017, July 31). About the Space Station Solar Arrays [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/mission_pages/station/structure/elements/solar_arrays-about.html</nowiki></ref> The Russian lamps, however, used xenon technology,<ref name=":4" /> which is anywhere from ½ to 10 times less efficient than modern LEDs in terms of lumens generated per watt.<ref>Luminous efficacy. (2019). In ''Wikipedia''. Retrieved from <nowiki>https://en.wikipedia.org/w/index.php?title=Luminous_efficacy&oldid=903091057</nowiki></ref> More efficient lighting could therefore theoretically reduce the energy requirements to somewhere between 11-54 kW, a much more feasible range considering the power output of the ISS. |
| − | Given the mass requirements of a plant-based system, such methods are perhaps better suited to future habitats on Mars. In-situ resource utilization techniques will allow colonists to avoid shipping large quantities of water, soil, or oxygen, all of which can be obtained on site.<ref name=":3" /> Hydroponic and aeroponic systems provide alternatives to any complications posed by the use of Mars regolith in a soil-based system, although this soil does contain all the nutrients necessary for plant survival once existing perchlorates are removed.<ref>Jordan, G. (2015, October 5). Can Plants Grow with Mars Soil? [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/feature/can-plants-grow-with-mars-soil</nowiki></ref> | + | Given the mass requirements of a plant-based system, such methods are perhaps better suited to future habitats on Mars. [[In-situ resource utilization]] techniques will allow colonists to avoid shipping large quantities of water, soil, or oxygen, all of which can be obtained on site.<ref name=":3" /> [[Hydroponics|Hydroponic]] and [[Aeroponics|aeroponic]] systems provide alternatives to any complications posed by the use of Mars [[regolith]] in a soil-based system, although this soil does contain all the nutrients necessary for plant survival once existing [[Perchlorate|perchlorates]] are removed.<ref>Jordan, G. (2015, October 5). Can Plants Grow with Mars Soil? [Text]. Retrieved August 16, 2019, from NASA website: <nowiki>http://www.nasa.gov/feature/can-plants-grow-with-mars-soil</nowiki></ref> |
==Airflow Complications in Microgravity Environment== | ==Airflow Complications in Microgravity Environment== | ||
| − | Regardless of whether artificial or biological systems provide the means for CO<sub>2</sub> scrubbing aboard a spacecraft, managing CO<sub>2</sub> concentrations in space presents several unique challenges.<ref name=":6" /> On Earth, gases flow and settle according to density differences, but this does not occur in microgravity. Special | + | Regardless of whether artificial or biological systems provide the means for CO<sub>2</sub> scrubbing aboard a spacecraft, managing CO<sub>2</sub> concentrations in space presents several unique challenges.<ref name=":6" /> On Earth, gases flow and settle according to density differences, but this does not occur in microgravity. Special ventilation equipment is required to ensure the CO<sub>2</sub> reaches appropriate intake areas, even if those intakes are biological in nature. Similarly, freshly scrubbed air must be distributed about the craft. These ventilation systems naturally require a certain degree of maintenance to avoid performance degradation due to the accumulation of dust or other debris. |
Similarly, crew members are rarely distributed about the spacecraft in a uniform fashion, meaning that pockets of higher CO<sub>2</sub> concentrations will develop where several crew members are working together, or when one is sleeping in the same space for several hours at a time. Furthermore, the volumetric characteristics of a transport spacecraft will regularly vary depending on the number of crew members, the shape and stowage position of cargo, the movement of that cargo obstructing airflow through passages, etc. All of these factors must be accounted for when installing and maintaining ventilation hardware. | Similarly, crew members are rarely distributed about the spacecraft in a uniform fashion, meaning that pockets of higher CO<sub>2</sub> concentrations will develop where several crew members are working together, or when one is sleeping in the same space for several hours at a time. Furthermore, the volumetric characteristics of a transport spacecraft will regularly vary depending on the number of crew members, the shape and stowage position of cargo, the movement of that cargo obstructing airflow through passages, etc. All of these factors must be accounted for when installing and maintaining ventilation hardware. | ||
| + | |||
| + | ==See also== | ||
| + | |||
| + | *[[Life support]] | ||
| + | *[[In-situ resource utilization]] | ||
==References== | ==References== | ||
* | * | ||
| − | + | [[Category:Air]] | |
Latest revision as of 16:46, 13 September 2019
volunteer for The Mars Society
It is licensed under Creative Commons BY-SA 3.0 and may be freely shared, but must include this attribution.
A steady supply of oxygen alone is insufficient to keep astronauts breathing. While the intake of oxygen is essential for respiration, the by-product of this respiration is carbon dioxide (CO2), a toxic gas which, depending on the extent of its concentration, can cause symptoms varying from mild discomfort to death. In the sealed environment of a spacecraft or a Mars habitat, this gas must be carefully monitored and removed from the air in a process called CO2 scrubbing. Spacecraft have until now used a variety of artificial means to accomplish this, although plant-based air recycling is a theoretical possibility which has been tested in some experimental settings.
Contents
Dangers of Excessive CO2 Concentration
A human being exhales approximately one kilogram of CO2 per day.[1] The concentration of this gas aboard the International Space Station averages about 0.4%, ten times that of Earth's atmosphere.[2] At these levels, side effects vary from person to person, with some astronauts unaffected and others experiencing headaches and irritability. At higher levels, however, CO2 can quickly become toxic:
| CO2 Concentration | Symptoms |
|---|---|
| 1% | Drowsiness |
| 3% | Impaired hearing, increased heart rate and blood pressure, stupor |
| 5% | Shortness of breath, headache, dizziness, confusion |
| 8% | Unconsciousness, muscle tremors, sweating |
| >8% | Death |
NASA has set strict limits of acceptable CO2 concentrations on spacecraft. The longer the duration of the flight, the lower the permissible maximum: for example, while allowing a maximum of 2% for a one-hour period, NASA recommends that the concentrations not exceed 0.5% over a 1000-day stay[1] (an as-yet hypothetical duration, as the record for longest consecutive stay in space at the time of writing is held by Russian cosmonaut Valery Polyakov at 438 days[4]). The proportionate decrease in concentration acceptability in accordance with duration accounts for the fact that the longer the period of time, the higher the likelihood of CO2-induced behavioral changes negatively affecting missions requiring close personal contact in confined spaces.
Moderating CO2 levels over such a long period of time naturally implies weight and power demands, yet NASA has adopted more stringent regulations than the Navy, which allows up to 2.5% concentrations for submarine personnel over a 24-hour period.[1] While both types of crews operate in a confined area with an artificially-regulated atmosphere, repairing a CO2 removal system in space constitutes a potentially more complex task where one cannot simply surface in the event of an unsuccessful attempt brought on by concentration difficulties. Hypothetically, a well-developed Mars colony could relax such limitations slightly once it is large enough to contain multiple fail-safes and back-ups, thereby reducing the energy demands on the colony as a whole. Even so, the discomfort caused by extended exposure to high CO2 concentrations means that maintaining them at near-Earth levels will likely remain a high priority.
In the days of interplanetary travel before colonists establish a self-sustaining colony, missions could also simply screen crews for CO2-resistant individuals.[2] As mentioned above, the effects of elevated CO2 concentrations aboard the ISS have affected astronauts to differing degrees—because higher permissible levels of CO2 mean less demand on scrubbing systems, ensuring crews consist only of members whose performance remains unchanged in the presence of increased concentrations could provide a straightforward way to reduce mission overhead.
Artificial CO2 Scrubbers
To date, spacecraft have regulated CO2 concentrations through artificial means. The simplest way to accomplish this consists of separating the CO2 molecules from the rest of the air and then either venting the toxic gas into space or storing it in a less harmful form.[5] Two types of systems can achieve this separation: chemical scrubbers and adsorbent scrubbers.[1]
Chemical Systems
These systems scrub CO2 from the air by bringing it into contact with a substance which attaches itself to the CO2 in a chemical reaction. Scrubbers can accomplish this with soda lime (a mixture of chemicals including calcium hydroxide, sodium hydroxide, and potassium hydroxide), amines (a derivative of ammonia), or potassium superoxide (a substance used in firefighter and mining rebreathers), but the low molecular weight and corresponding transport costs of lithium hydroxide (LiOH) has led to its popularity aboard spacecraft.[3][5] Bringing CO2 into contact with LiOH creates lithium carbonate (Li2CO3) and water through the following reaction:
CO2 (g) + 2LiOH (s) → Li2CO3 (s) + H2O (l)
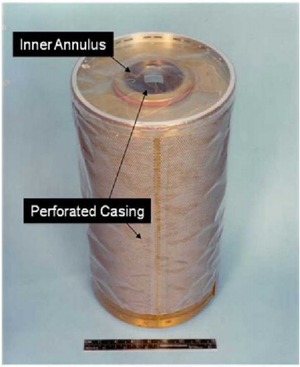
The conversion to Li2CO3 is irreversible, meaning that the canisters used for the reaction must be replaced once all of the LiOH is consumed.[6]
Apollo 13 provides perhaps the most famous instance of complications arising due to this replacement process:[3] an explosion in the command module forced all three crew members into the lunar module, whose air recycling system was designed for only two astronauts. The lunar module's LiOH canisters quickly became saturated, and the crew had to improvise extensive modifications using hoses, socks, plastic bags, and duct tape in order to adapt the command module's square canisters to the round lunar module system.
In addition to the Apollo program, LiOH canisters constituted the primary CO2 removal system during Mercury, Gemini, and Space Shuttle missions.[1] One canister acted as the primary scrubber, with another in place as a backup; as the primary canister became depleted, the backup took over and the primary was replaced, with this alternating cycle repeating as many times as necessary until the crew returned to Earth. The longer the mission, the more canisters required.
Scrubbing the CO2 which a single crew member exhales over the course of a day requires roughly 1.5 kilograms of LiOH.[1] Modern LiOH canisters used by NASA contain 3.0 kilograms of LiOH pellets, and the water created during the chemical reaction brings the weight of a depleted canister to around 4.0 kilograms.[1][6] Russian Soyuz canisters are almost twice as large. This weight, along with the size of the canisters (approximately 6 liters in volume), adds substantial considerations not only to the cost of launching from Earth, but also to returning, since the canisters are not jettisoned into space.[1]
Because the number of required canisters increases in direct proportion to mission length, regenerative CO2 scrubbers become more cost effective than a system based on consumable LiOH after a period of 20-30 days.[7] Skylab and Mir still employed LiOH canisters in a supplementary capacity, as does the International Space Station, which maintains a minimum contingency reserve capable of sustaining 3 crew members for 15 days, as well as a stockpile from which docking craft can draw when payload requirements restrict the amount available for their own scrubbing needs.[1] The primary system employed on the ISS, however, utilizes regenerative filtering technology.
Adsorption Systems
Adsorption differs from the more well-known absorption process in that the latter involves a fluid permeating the entire volume of a liquid or solid (like a sponge soaking up water), while the former consists of a substance adhering only to the surface (like dust clinging to an air filter).[8] Adsorption CO2 scrubbers pass air over a bed of material which causes the CO2 molecules to adhere to it temporarily before being vented into space. The process can then be repeated, meaning that adsorption systems, in contrast to chemical LiOH scrubbers, are reusable in nature. These systems have served as the primary CO2 scrubbers on Skylab, Mir, and the International Space Station.
CDRA
Two adsorption-based Carbon Dioxide Removal Assembly (CDRA) systems serve the US portion of the ISS.[3] The CDRA uses crystals of silicon dioxide and aluminum dioxide called zeolites, which arrange themselves in a pattern similar to a small screen. Depending on the type of zeolite, different molecules become trapped in these sieves: the CDRA employs one type of zeolite bed to adsorb water in a desiccant bed, and another to adsorb CO2. Each bed becomes saturated after adsorbing a certain amount of its respective molecule, but unlike the non-regenerative LiOH canisters, the CDRA beds can desorb the attached substances and then be reused.
The CDRA system utilizes two sets of these two-bed combinations in alternating conjunction with one another, with each set containing one desiccant bed and one CO2 adsorption bed. At any given time, one of these two beds in a set is adsorbing while the other is desorbing, and vice versa for the other set. That is, if the desiccant bed of one set is currently adsorbing water, then the CO2 bed in the same set as well as the desiccant bed of the opposite set will be desorbing.[6] In this way, half of the system is always removing water and CO2 while the other half is regenerating its capability to do so.
Air first passes over a desaturated desiccant bed, removing moisture which can inhibit the function of the adsorbing CO2 bed in the next step. After the CO2 is removed, the dry, scrubbed air passes over a saturated desiccant bed, which re-humidifies it before returning it to the cabin. Once the CO2 bed is fully saturated, it can then be vented directly into space or combined with hydrogen gas to form water and methane gas using the onboard Sabatier system. When the previously adsorbing beds become saturated, air is pumped the opposite way through the system while the saturated beds desorb and the now-desaturated beds begin to adsorb again.
In theory, this process can continue indefinitely and therefore provides an attractive alternative to chemical scrubbers. Even so, CDRA has suffered its share of complications.[6] The zeolite of the desiccant and CO2 adsorption beds takes the form of loose pellets, and because the process necessitates blowing air over these beds, early implementations experienced problems with the pellets being blown off the beds and into air valves. Zeolite dust has also accumulated to the point where airflow became restricted beyond functional levels, and electrical shorts have caused additional mechanical problems. These issues have been addressed through various design revisions,[1][6] but demonstrate the drawbacks inherent in a complicated system such as CDRA, despite the advantages of its regenerative nature.
Amine-based systems
While CDRA uses zeolite as an adsorbent, amine has also shown potential in past and future systems. CDRA's Russian counterpart, the Vozdukh system, removes CO2 in much the same fashion as CDRA, but uses a 3-bed amine system instead of CDRA's 2-bed zeolite configuration.[1][6] This system proved quite effective on the ISS, supporting a crew of 6 and and removing 120 liters of CO2 per hour. Degradation of Vozdukh's functional capacity, however, meant that CDRA eventually took over primary CO2 removal duties.
NASA's Orion crew vehicle also plans to use a more efficient implementation of this amine-based technology.[1][9] As recently as April 2019, the ISS tested a Thermal Amine Scrubber which includes, among other features, a desiccant wheel serving as a second de-humidification step before dried air comes into contact with the adsorbing amine bed.[10][11] This will reduce the amount of water vapor which is vented overboard with the waste CO2.
Other Artificial Regenerative Options
The CO2 scrubbing options discussed in this section focus on removing the toxic gas from the spacecraft, but several options exist which convert the CO2 into another substance of value. These include:
- The Sabatier reaction to produce water and methane.
- The Bosch reaction to produce water and carbon.
- The reverse water-gas shift reaction to produce water and carbon monoxide.
- Solid oxide electrolysis to produce carbon monoxide and oxygen.
The last of these processes will be tested on Mars aboard the Mars 2020 MOXIE (Mars Oxygen ISRU Experiment) project.[12] In contrast to spacecraft, where CO2 scrubbers seek to isolate quantities of the gas which are relatively small in comparison to the overall air content, Mars has an atmosphere almost entirely (96%) composed of CO2. This means that with the help of ISRU techniques, a vast supply of oxygen for both breathing and rocket fuel can be obtained without shipping it from Earth. Should the MOXIE prototype prove successful, scaled-up versions 100 times its size could save billions of dollars per launch.[13]
MOXIE will collect atmospheric CO2, pressurize it, and then electrochemically split it in a process similar to running a fuel cell in reverse.[12] The intention of the experiment at this stage is not to build up any stockpile of oxygen on the planet, but merely to serve as a proof of concept for future missions—all products of the reaction will be released back into the atmosphere after the oxygen is checked for purity and the gases are filtered in accordance with planetary protection requirements.[13] The thermal requirements of the system are extensive, but it has the advantage of being able to produce an unlimited amount of oxygen without the addition of any other off-planet substances.[14]
Biological CO2 Scrubbers
The chief advantage of a biological recycling system over a mechanically-sustained one is simple: plants don't break. Where a machine might suddenly cease to function and require expensive parts shipped from Earth, nature has largely optimized self-repair via evolution. Given the relatively simple level of technology involved in proper lighting, plumbing, and pumps, and provided that no pests or insects are introduced to the sterile conditions of space, plants could potentially be far more reliable than artificial CO2 scrubbers.[15] Furthermore, the right plants could also double as a food source for astronauts or colonists.
Previous Experiments
Several closed-ecosystem experiments conducted on Earth have investigated the viability of a plant-sustained air recycling system. In the 1970s, the BIOS-3 facility in Siberia found that 8m2 of Chlorella algae could maintain the 02/CO2 balance for one individual in a 315 m3 environment.[16]
The Biosphere 2 project in Arizona attempted a larger-scale project over the course of 2 years, sealing 8 participants in a 180,000 m3 facility with an artificial ecosystem.[17] The facility operated in mostly closed-loop conditions, but was exposed to natural sunlight. Daily variance in this light caused CO2 levels to fluctuate substantially according to the relative activity of photosynthesis: while CO2 concentrations dropped during the day, they rose sharply at night when respiration predominated. Seasonal variation was similarly evident, with levels rising in low-light winter months and on cloudy days, while longer sun exposures corresponding with summer months resulted in lower concentrations. December, for example, averaged CO2 levels over twice as high as those in June, with the two months differing by up to 5 hours of sun exposure at their extreme ends.
The project utilized various methods to attenuate the effects of this variance, including CO2 sequestration via calcium carbonate precipitation, lowering nighttime temperatures to reduce respiration, and storing trimmed biomass in dry areas to slow its decomposition. Notably, an unanticipated, steady decline in oxygen levels—which required the artificial injection of fresh oxygen after dropping to a point where team members were suffering symptoms of altitude sickness—was attributed to additional microbial respiration from organically-enriched soil.[18] Unsealed concrete in the habitat absorbed the CO2 released by this respiration, and therefore prevented its re-conversion into oxygen via photosynthesis.
Potential for Future Missions
With further investigation, these plant-based systems could potentially fulfill the air-recycling needs of future space expeditions. Biological means have yet to feature as the primary form of CO2 scrubbing for spacecraft due to a variety of reasons including mass constraints, the number of plants required per individual, and the energy costs of optimized lighting and air conditioning.[7][17] The International Space Station, for example, has plants aboard, but not in the quantities necessary to have any appreciable impact on CO2 concentrations.[2]
Yet, the above experiments hint at the possibility of an exclusively bioregenerative system. The ISS has a habitable volume (388 m3) slightly larger than the 315 m3 environment of the successful BIOS-3 experiment, and a pressurized volume on the order of 3 times as large at 932 m3.[19] The BIOS-3 experiments supported up to 3 individuals for 180 days,[16] meaning that the ISS could theoretically recycle air for a crew of at least 8.[15]
The algae in these experiments used 18 kW of artificial lighting per person; extrapolating this to 108 kW for a crew of six on the ISS—which could not rely on natural light alone due to regularly being in darkness—would consume the majority of the 120 kW generated by the station's solar arrays.[15][20] The Russian lamps, however, used xenon technology,[15] which is anywhere from ½ to 10 times less efficient than modern LEDs in terms of lumens generated per watt.[21] More efficient lighting could therefore theoretically reduce the energy requirements to somewhere between 11-54 kW, a much more feasible range considering the power output of the ISS.
Given the mass requirements of a plant-based system, such methods are perhaps better suited to future habitats on Mars. In-situ resource utilization techniques will allow colonists to avoid shipping large quantities of water, soil, or oxygen, all of which can be obtained on site.[17] Hydroponic and aeroponic systems provide alternatives to any complications posed by the use of Mars regolith in a soil-based system, although this soil does contain all the nutrients necessary for plant survival once existing perchlorates are removed.[22]
Airflow Complications in Microgravity Environment
Regardless of whether artificial or biological systems provide the means for CO2 scrubbing aboard a spacecraft, managing CO2 concentrations in space presents several unique challenges.[6] On Earth, gases flow and settle according to density differences, but this does not occur in microgravity. Special ventilation equipment is required to ensure the CO2 reaches appropriate intake areas, even if those intakes are biological in nature. Similarly, freshly scrubbed air must be distributed about the craft. These ventilation systems naturally require a certain degree of maintenance to avoid performance degradation due to the accumulation of dust or other debris.
Similarly, crew members are rarely distributed about the spacecraft in a uniform fashion, meaning that pockets of higher CO2 concentrations will develop where several crew members are working together, or when one is sleeping in the same space for several hours at a time. Furthermore, the volumetric characteristics of a transport spacecraft will regularly vary depending on the number of crew members, the shape and stowage position of cargo, the movement of that cargo obstructing airflow through passages, etc. All of these factors must be accounted for when installing and maintaining ventilation hardware.