Difference between revisions of "In-situ resource utilization"
| (37 intermediate revisions by 3 users not shown) | |||
| Line 26: | Line 26: | ||
The use of local resources is called '''in-situ resource utilization''' or ISRU. This concept is critical to the survival of an [[Foundation of an Autonomous Colony|autonomous]] or [[Semi-autonomous colony|semi-autonomous]] [[settlement]]. The [[ISRU timeline]] is very rapid for most settlement plans since the initial Zubrin proposal of MArs Direct. | The use of local resources is called '''in-situ resource utilization''' or ISRU. This concept is critical to the survival of an [[Foundation of an Autonomous Colony|autonomous]] or [[Semi-autonomous colony|semi-autonomous]] [[settlement]]. The [[ISRU timeline]] is very rapid for most settlement plans since the initial Zubrin proposal of MArs Direct. | ||
| − | ==Inputs== | + | |
| + | Resources(inputs) are processed to create products(outputs). These outputs can become inputs to other processes used to create new, more complex outputs. Eventually, the outputs can be re-used by recycling, reducing the needs for new resources. However, losses are always present is systems, making the acquisition of new resources a continuous requirement. The presence of numerous resources on Mars is part of what makes it an interesting target for colonization and settlement. | ||
| + | |||
| + | Production processes require energy to operate and must be housed in [[Production|production facilities]]. | ||
| + | ==Resources (Inputs)== | ||
===Atmosphere=== | ===Atmosphere=== | ||
| Line 53: | Line 57: | ||
*[[Sulfates]] | *[[Sulfates]] | ||
*[[Nitrates]] are sources of nitrogen for plants and industrial processes, ammonia and explosives. Nitrates were discovered on Mars by the [[Curiosity]] rover in 2015. | *[[Nitrates]] are sources of nitrogen for plants and industrial processes, ammonia and explosives. Nitrates were discovered on Mars by the [[Curiosity]] rover in 2015. | ||
| − | *[[Salts]]. (Mg,Na)SO4, NaCl, and (Mg,Ca)CO3. Magnesium, Calcium, Sodium, | + | *[[Salts]]. (Mg,Na)SO4, NaCl, and (Mg,Ca)CO3. Magnesium, Calcium, Sodium, Lithium, Chlorine. Practically all minerals and elements can be found in the form of salts. Sodium chloride (NaCl) is the most common salt, and is essential for life. Chlorides are likely to be abundant on Mars.<ref>Wikipedia- Chlorides on Mars[https://en.wikipedia.org/wiki/Chloride-bearing_deposits_on_Mars]</ref> |
*[[Thorium]] (Th) concentrations have been identified by JPL on Mars, this is the preferred fuel in a number of Molten Salt Reactor designs. <ref name=":12">Map of Martian Thorium at Mid-Latitudes, JPL '' Map of Martian Thorium at Mid-Latitudes '', https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.</ref> | *[[Thorium]] (Th) concentrations have been identified by JPL on Mars, this is the preferred fuel in a number of Molten Salt Reactor designs. <ref name=":12">Map of Martian Thorium at Mid-Latitudes, JPL '' Map of Martian Thorium at Mid-Latitudes '', https://www.jpl.nasa.gov/spaceimages/details.php?id=PIA04257, March 2003.</ref> | ||
| + | *[[Fluorine]] (F) has been found in small quantities on Mars. It is an important resource if people wish to warm Mars with [[Super Greenhouse Gases]]. | ||
===Energy=== | ===Energy=== | ||
| Line 63: | Line 68: | ||
*[[Photovoltaics|Solar energy]] is a very variable energy source on Mars, unless some form Space Solar energy is used. It requires some form of [[Energy storage|energy storage]] in the settlement or supplementary energy sources, most likely nuclear. | *[[Photovoltaics|Solar energy]] is a very variable energy source on Mars, unless some form Space Solar energy is used. It requires some form of [[Energy storage|energy storage]] in the settlement or supplementary energy sources, most likely nuclear. | ||
*[[Nuclear power|Nuclear energy]] is a stable energy source and could be used extensively on Mars. The availability of nuclear fuel on Mars needs to be explored and usable ore deposits found for a self sustainable settlement. | *[[Nuclear power|Nuclear energy]] is a stable energy source and could be used extensively on Mars. The availability of nuclear fuel on Mars needs to be explored and usable ore deposits found for a self sustainable settlement. | ||
| − | *[[Geothermal energy]] may be available in some martian sites. | + | *[[Geothermal energy]] may be available in some martian sites. There is evidence of Martian Vulcanism in the last 50,000 to 200,000 years, so there is almost certainly geothermal energy in some areas. |
| + | *[[Wind turbine|Wind power]] has been discussed for some applications. However, Mars' air pressure is so low that this will provide negligible power compared to other sources. If Mars' atmosphere were to be thickened, it might become a viable energy source as solar power might then become weaker. Note that wind energy fundamentally comes from the Sun, and Mars gets about half the solar energy than the Earth does. So it is likely that wind power will be less useful on Mars, even if it is given a thicker atmosphere than currently. Also note that the extremely small dust particles (fines), will be hard on mechanical moving parts. | ||
==Processes== | ==Processes== | ||
| Line 88: | Line 94: | ||
*[[Sabatier/Water Electrolysis Process]] | *[[Sabatier/Water Electrolysis Process]] | ||
*[[Fischer-Tropsch reaction|Fischer-Tropsch Reaction]] | *[[Fischer-Tropsch reaction|Fischer-Tropsch Reaction]] | ||
| − | *[[Electrolysis]] | + | *Water [[Electrolysis]] |
| + | *CO [[Carbon_dioxide_electrolysis]] | ||
| − | ===== | + | =====Alcohols===== |
*Methanol synthesis | *Methanol synthesis | ||
| − | *Formox process | + | *[[w:Formox_process|Formox process]]. For the production of Formaldehyde |
*MTO process | *MTO process | ||
*Dehydration | *Dehydration | ||
| Line 107: | Line 114: | ||
*Wacker process | *Wacker process | ||
*Vinyl acetate monomer | *Vinyl acetate monomer | ||
| − | *Polymerization | + | *[[Polymerization]] |
| − | *Hydroformylation | + | *[[Hydroformylation]] |
| − | *Hydrogenation | + | *[[Hydrogenation]] |
=====Direct ethylene glycol synthesis===== | =====Direct ethylene glycol synthesis===== | ||
| Line 120: | Line 127: | ||
=====Reduction===== | =====Reduction===== | ||
| − | Usually, but not exclusively, of a compound into individual elements. [[Iron ore]] into iron and oxygen, for example. | + | Usually, but not exclusively, a separation of a compound into individual elements. [[Iron ore]] into iron and oxygen, for example. |
===Biological processes=== | ===Biological processes=== | ||
| Line 127: | Line 134: | ||
*[[Biomethanisation]] | *[[Biomethanisation]] | ||
| − | ==Outputs== | + | ==Products (Outputs)== |
| + | |||
| + | =====Solids===== | ||
| + | |||
| + | *[[Sand]] is one of the most common building materials. Composition varies greatly. | ||
| + | *[[Gravel]] | ||
| + | *[[Cement]] is a binder used to link together elements such as blocks and bricks or gravel and sand to produce concrete. | ||
| + | *[[Concrete]] | ||
| + | *[[Sulphur Concrete]] uses molten sulphur (120 degrees C) to act as binder for concrete. | ||
| + | *[[StarCrete]] is a concrete made with starch, a bit of salt, & [[Regolith]]. | ||
| + | *[[Compressed regolith]] | ||
| + | *[[Glass]] is one of the most common building materials on Earth and should be common on Mars as well, since it has unique properties of low cost and transparency. Silica (SiO2), the main component of glass, is also the most common material in the Martian crust. | ||
| + | |||
| + | *[[Ceramics]] | ||
| + | |||
| + | *[[Fertilizer]] (N....) | ||
| + | |||
| + | *[[Biochar]] (Cx) | ||
| + | *[[Synthetic materials|Synthetic Polymers]] and [[Carbon fiber|carbon fiber]] precursors | ||
| + | **[[Polyethylene]] (PE) | ||
| + | **[[Polypropylene]] (PP) | ||
| + | **[[Polyester]] (PET) | ||
| + | **[[Polyvinyl Chloride|Polyvinyl chloride]] (PVC) | ||
| + | **Polyamides (Nylon) | ||
| + | **Polytetrafluoroethylene (PTFE,Teflon) | ||
| + | *[[Biomass]] | ||
| + | * | ||
| + | |||
| + | =====Metals===== | ||
| + | |||
| + | *[[Iron]] | ||
| + | *[[steel]] | ||
| + | *[[Aluminium|Aluminum]] | ||
| + | *[[Copper]] | ||
| + | *[[Nickel]] | ||
| + | *[[Silicon]] | ||
| + | |||
===Liquids=== | ===Liquids=== | ||
| − | *[[Water]]. Essential for life. It is also a common process reagent, an excellent coolant for industrial processes and a source of hydrogen and oxygen using electrolysis. On Mars it can also be used as a construction material or as radiation shielding. It can be condensed out of the atmosphere or extracted from the regolith. | + | *[[Water]] (H<sub>2</sub>O). Essential for life. It is also a common process reagent, an excellent coolant for industrial processes and a source of hydrogen and oxygen using electrolysis. On Mars it can also be used as a construction material or as radiation shielding. It can be condensed out of the atmosphere or extracted from the regolith. |
| − | *[[Ammonia]] | + | *[[Ammonia]] (NH<sub>3</sub>) |
*[[Hydrocarbons]] | *[[Hydrocarbons]] | ||
| − | ** | + | ** |
**Light olefins | **Light olefins | ||
**Light alkenes | **Light alkenes | ||
| Line 141: | Line 184: | ||
**Bio-oil | **Bio-oil | ||
*[[Alcool|Alcools]] | *[[Alcool|Alcools]] | ||
| − | **[[Ethanol]] | + | **[[Ethanol]] (C<sub>2</sub>H<sub>5</sub>OH) |
| − | **[[Methanol]] | + | **[[Methanol]] ([[Carbon|C]][[Hydrogen|H]]<sub>3</sub>[[Oxygen|O]]H) |
| + | *[[Acid|Acids]] | ||
| + | **[[Acetic acid]] (C<sub>2</sub>H<sub>4</sub>O<sub>2</sub>) | ||
| + | **[[Sulfuric acid]] (H<sub>2</sub>SO<sub>4</sub>) | ||
| + | **[[Aspirin|Salicylic acid]] | ||
* | * | ||
* | * | ||
| Line 149: | Line 196: | ||
===Gases=== | ===Gases=== | ||
| − | A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained | + | A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained by starting with oxygen and adding buffer gases to reach the desired proportions. Humans can breath pure oxygen at 1/5 atmospheric pressure, but adding buffer gases such as nitrogen or argon is desirable. It is likely that people will choose an air pressure less than one atmosphere for early settlements on Mars. Carbon dioxide is required for plants but must be kept to about 1000 ppm or less, as it rapidly becomes toxic at even low partial pressures. Carbon monoxide is even more toxic and must be removed from the settlement atmosphere. |
| − | *[[Oxygen]] ( | + | *[[Oxygen]] (O<sub>2</sub>) |
| − | *[[hydrogen]] ( | + | *[[hydrogen]] (H<sub>2</sub>) |
| − | *[[Nitrogen]] ( | + | *[[Nitrogen]] (N<sub>2</sub>) |
*[[Argon]] (Ar) | *[[Argon]] (Ar) | ||
| − | *[[Carbon dioxide|Carbon | + | *[[Carbon dioxide|Carbon dioxide]] (CO<sub>2</sub>) |
| − | *[[Carbon monoxide|Carbon | + | *[[Carbon monoxide|Carbon monoxide]] (CO) |
*[[Hydrocarbons]] | *[[Hydrocarbons]] | ||
| − | **[[Methane]] ( | + | **[[Methane]] (CH<sub>4</sub>) |
| − | **[[Ethylene]] | + | **[[Ethylene]] (C<sub>2</sub>H<sub>4</sub>) |
| − | **[[Propylene]] | + | **[[Propane|Propane (C<sub>3</sub>H<sub>8</sub>)]] |
| − | **[[ | + | **[[Butane]] (C<sub>4</sub>H<sub>10</sub>) |
| − | **[[ | + | **[[Propylene]] (C<sub>3</sub>H<sub>6</sub>) |
| + | **[[Acetylene]] (C<sub>2</sub>H<sub>2</sub>) | ||
| + | **[[Formaldehyde]] ( CH<sub>2</sub>O) | ||
| + | **[[Vinyl Chloride]] (C<sub>2</sub>H<sub>3</sub>Cl) | ||
* | * | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
[[Category:In-situ Resource Utilization]] | [[Category:In-situ Resource Utilization]] | ||
<references /> | <references /> | ||
Latest revision as of 11:37, 4 November 2024
The use of local resources is called in-situ resource utilization or ISRU. This concept is critical to the survival of an autonomous or semi-autonomous settlement. The ISRU timeline is very rapid for most settlement plans since the initial Zubrin proposal of MArs Direct.
Resources(inputs) are processed to create products(outputs). These outputs can become inputs to other processes used to create new, more complex outputs. Eventually, the outputs can be re-used by recycling, reducing the needs for new resources. However, losses are always present is systems, making the acquisition of new resources a continuous requirement. The presence of numerous resources on Mars is part of what makes it an interesting target for colonization and settlement.
Production processes require energy to operate and must be housed in production facilities.
Resources (Inputs)
Atmosphere
Main article: Atmospheric processing
Many of the elements and molecules in the atmosphere can be utilized. Condensation, followed by distillation, are often used to extract resources. The atmosphere is first cooled to a liquid or solid state. This is distilled at precise temperatures in order to separate the elements and molecules.
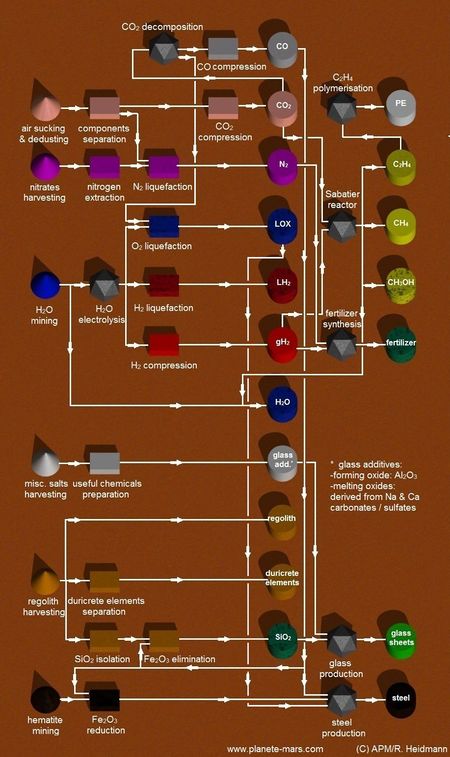
- Carbon dioxide (CO2) composes 96% of the martian atmosphere. Carbon dioxide is the main source of carbon, used for fuel production (CH4) and an essential element for life. Carbon dioxide also serves as a source of oxygen for the settlement atmosphere and as the oxidizer in bi-propellant fuels.
- Nitrogen composes 2% of martian atmosphere. Nitrogen is used by plants and is part of a breathable atmosphere. Its concentration on Earth is 78% of the atmosphere.
- Argon is an inert gas, useful in some industrial processes as an inert atmosphere and may be used as propellant in Electric Propulsion of spaceships.2% of martian atmosphere
- Water (H2O) is the main source of hydrogen and oxygen. Oxygen is required for the settlement atmosphere and hydrogen is used for fuel production (CH4) and for the synthesis of hydrocarbons, the building blocks for life.
Lithosphere (surface)
Main article: Mining
Minerals in the crust of Mars must be mined and processed to be useful. The upper layer of Mars surface is called the Regolith. It is a mixtures of materials of various interest.
- Water can be gathered in a variety of ways. It is available in the form of water ice or as hydrated minerals.
- Silicates (SiO2) are useful for the production of glass and building materials. It is one of the main components of the martian planetary crust.
- Iron ore ( Hematite:Fe2O3) or (Magnetite: Fe3O4) is a source of iron and steel, as well as oxygen or CO2, depending on the process used.
- Alumina (Al2O3) is the source of aluminium. Processing also produces CO2, oxygen or water, depending on the process used.
- Calcium carbonate (CaCO3) is used for concrete production. Carbonates are also a potential source of carbon for carbohydrates. Sodium carbonates are used in glass production. Carbonates are available on Mars.[1]
- Sulfates
- Nitrates are sources of nitrogen for plants and industrial processes, ammonia and explosives. Nitrates were discovered on Mars by the Curiosity rover in 2015.
- Salts. (Mg,Na)SO4, NaCl, and (Mg,Ca)CO3. Magnesium, Calcium, Sodium, Lithium, Chlorine. Practically all minerals and elements can be found in the form of salts. Sodium chloride (NaCl) is the most common salt, and is essential for life. Chlorides are likely to be abundant on Mars.[2]
- Thorium (Th) concentrations have been identified by JPL on Mars, this is the preferred fuel in a number of Molten Salt Reactor designs. [3]
- Fluorine (F) has been found in small quantities on Mars. It is an important resource if people wish to warm Mars with Super Greenhouse Gases.
Energy
Main article: Energy
Energy is required to carry out ISRU. There are three known sources of energy on Mars: the Sun, Mars' crust and nuclear fission. Energy may be stored in a variety of ways for when the sources are not available.
- Solar energy is a very variable energy source on Mars, unless some form Space Solar energy is used. It requires some form of energy storage in the settlement or supplementary energy sources, most likely nuclear.
- Nuclear energy is a stable energy source and could be used extensively on Mars. The availability of nuclear fuel on Mars needs to be explored and usable ore deposits found for a self sustainable settlement.
- Geothermal energy may be available in some martian sites. There is evidence of Martian Vulcanism in the last 50,000 to 200,000 years, so there is almost certainly geothermal energy in some areas.
- Wind power has been discussed for some applications. However, Mars' air pressure is so low that this will provide negligible power compared to other sources. If Mars' atmosphere were to be thickened, it might become a viable energy source as solar power might then become weaker. Note that wind energy fundamentally comes from the Sun, and Mars gets about half the solar energy than the Earth does. So it is likely that wind power will be less useful on Mars, even if it is given a thicker atmosphere than currently. Also note that the extremely small dust particles (fines), will be hard on mechanical moving parts.
Processes
Inputs need to be processed to create outputs
Mechanical processes
- Compression of gases increases their density and can change conditions to allow for phase changes that can be used for gas separation.
- Heating and cooling can also be used to accomplish phase changes in various substances.
- Crushing and milling. These are mechanical processes that break minerals down to individual crystals for separation and materials handling. Complex minerals such as basalts, granites or ores can be broken down for separation
- Separation Mechanical, centrifugal,
- Flotation,
- Distillation,
- Condensation
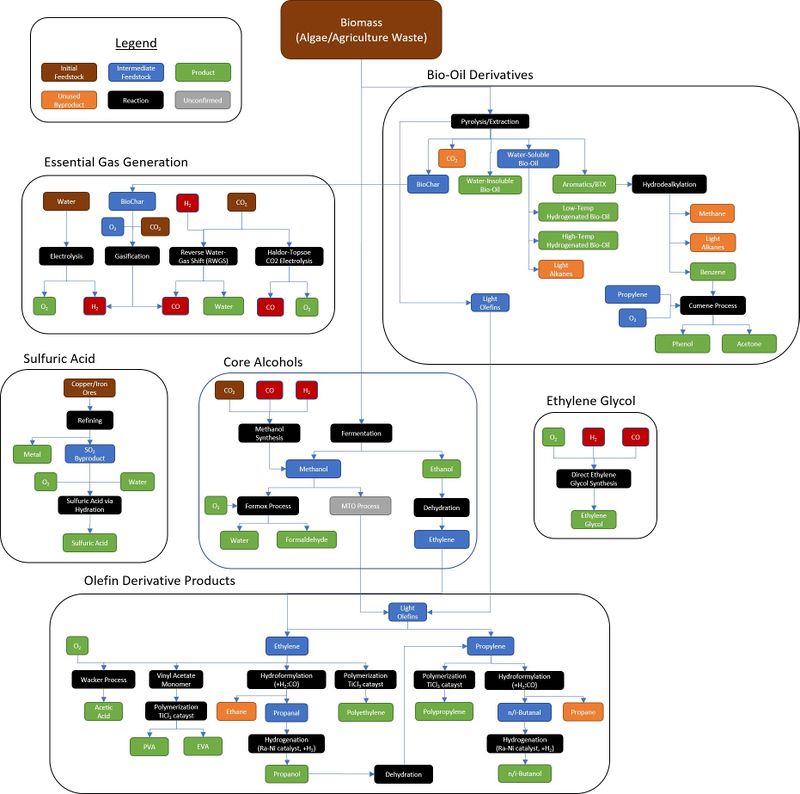
Chemical processes
Gases
- Gasification
- Reverse Water-Gas Shift Reaction
- Haldor Topsoe CO2 electrolysis
- Sabatier/Water Electrolysis Process
- Fischer-Tropsch Reaction
- Water Electrolysis
- CO Carbon_dioxide_electrolysis
Alcohols
- Methanol synthesis
- Formox process. For the production of Formaldehyde
- MTO process
- Dehydration
Bio-oil derivatives
- Pyrolysis/extraction
- Hydrodealkylation
- Cumene process
Olefin derivatives
- Wacker process
- Vinyl acetate monomer
- Polymerization
- Hydroformylation
- Hydrogenation
Direct ethylene glycol synthesis
Hydrocarbon synthesis
Hydrocarbons can be manufactured by combining and carbon through a variety of reactions
Silicone Synthesis
Reduction
Usually, but not exclusively, a separation of a compound into individual elements. Iron ore into iron and oxygen, for example.
Biological processes
Products (Outputs)
Solids
- Sand is one of the most common building materials. Composition varies greatly.
- Gravel
- Cement is a binder used to link together elements such as blocks and bricks or gravel and sand to produce concrete.
- Concrete
- Sulphur Concrete uses molten sulphur (120 degrees C) to act as binder for concrete.
- StarCrete is a concrete made with starch, a bit of salt, & Regolith.
- Compressed regolith
- Glass is one of the most common building materials on Earth and should be common on Mars as well, since it has unique properties of low cost and transparency. Silica (SiO2), the main component of glass, is also the most common material in the Martian crust.
- Fertilizer (N....)
- Biochar (Cx)
- Synthetic Polymers and carbon fiber precursors
- Polyethylene (PE)
- Polypropylene (PP)
- Polyester (PET)
- Polyvinyl chloride (PVC)
- Polyamides (Nylon)
- Polytetrafluoroethylene (PTFE,Teflon)
- Biomass
Metals
Liquids
- Water (H2O). Essential for life. It is also a common process reagent, an excellent coolant for industrial processes and a source of hydrogen and oxygen using electrolysis. On Mars it can also be used as a construction material or as radiation shielding. It can be condensed out of the atmosphere or extracted from the regolith.
- Ammonia (NH3)
- Hydrocarbons
- Alcools
- Acids
- Acetic acid (C2H4O2)
- Sulfuric acid (H2SO4)
- Salicylic acid
Gases
A breathable atmosphere is a basic requirement for life. It is also needed for heat transfer from people, plants and animals. It is obtained by starting with oxygen and adding buffer gases to reach the desired proportions. Humans can breath pure oxygen at 1/5 atmospheric pressure, but adding buffer gases such as nitrogen or argon is desirable. It is likely that people will choose an air pressure less than one atmosphere for early settlements on Mars. Carbon dioxide is required for plants but must be kept to about 1000 ppm or less, as it rapidly becomes toxic at even low partial pressures. Carbon monoxide is even more toxic and must be removed from the settlement atmosphere.
- Oxygen (O2)
- hydrogen (H2)
- Nitrogen (N2)
- Argon (Ar)
- Carbon dioxide (CO2)
- Carbon monoxide (CO)
- Hydrocarbons
- Methane (CH4)
- Ethylene (C2H4)
- Propane (C3H8)
- Butane (C4H10)
- Propylene (C3H6)
- Acetylene (C2H2)
- Formaldehyde ( CH2O)
- Vinyl Chloride (C2H3Cl)