Difference between revisions of "Water"
| Line 95: | Line 95: | ||
Power sources which rely on heat engines (such as [[nuclear power]]) require a heat sink to provide the heat differential required for the engine to run. Water or Ice make good materials for this heat sink as they are dense and have high thermal mass. The ice of [[korolev|Korolev Crater]] has been suggested as a potential heat sink sufficient to provide for colony scale power generation. | Power sources which rely on heat engines (such as [[nuclear power]]) require a heat sink to provide the heat differential required for the engine to run. Water or Ice make good materials for this heat sink as they are dense and have high thermal mass. The ice of [[korolev|Korolev Crater]] has been suggested as a potential heat sink sufficient to provide for colony scale power generation. | ||
| − | [[Deuterium]] from martian water may provide a source of fusion fuel for future energy production. Note that deuterium is | + | [[Deuterium]] from martian water may provide a source of fusion fuel for future energy production. Note that deuterium is about six times more concentrated on Mars than on Earth, and may form a viable export. |
[[Methanol]] and [[methane]] may be used to feed [[Biological_reactors|methanotrophs]] to produce food or other biologically produced industrial materials. | [[Methanol]] and [[methane]] may be used to feed [[Biological_reactors|methanotrophs]] to produce food or other biologically produced industrial materials. | ||
Revision as of 16:39, 14 March 2023
Water is a chemical compound consisting of a single oxygen atom bonded to two hydrogen atoms (chemical symbols: H2O). Water is essential to all known forms of life, and its unique properties make it invaluable for most industrial processes. While water in the liquid phase is abundant on Earth, its icy deposits on Mars make it into a critical resource to be treasured.
Contents
Evidence for water on Mars
Starting in 2004, the evidence of the presence of water on Mars has been mounting.
Past liquid water
Mars shows evidence of extensive liquid water flowing on its surface in the past and it is the focus of many Mars missions to find out how this water has leaked away over the millennia.
The 1996 Mars Pathfinder mission discovered plentiful evidence that its landing site, Ares Vallis, was once the bottom of a huge valley system eroded by ancient water.
In 2004, the Opportunity rover discovered geological markers - stratification and cross-bedding - near its landing site which pointed to significant flows of water at some time in Mars' history.[1]
The Mars Express Orbiter used imaging spectroscopy to detect hydrated minerals in 2005, strong evidence that surface water was once present in large amounts and for a long duration.[2]
Further support for the historic existence of flowing water comes from the first observations made by NASA's Mars Reconnaissance Orbiter (launched in 2005) where the High Resolution Imaging Science Experiment (HiRISE) camera spotted small fractures and cracks in the Martian canyon, Candor Chasma. The cracks analyzed show signs of mineral alteration in the rock exposed - a sign that liquid water once flowed through these sub-surface pipes.
- "What caught my eye was the bleaching or lack of dark material along the fracture. That is a sign of mineral alteration by fluids that moved through those joints. It reminded me of something I had seen during field studies in Utah, that is light-tone zones, or 'haloes,' on either side of cracks through darker sandstone" - Dr. Chris Okubo, a geologist at the University of Arizona, Tucson.
Although this is a sign that the liquid water has since disappeared from these cracks and fractures in the canyon rock, it is interesting to find evidence for ancient water in abundance.
Current water ice
Today, water on Mars appears to be concentrated in Martian polar ice, suggesting Mars may once have had a warmer climate, slowly cooling as the atmosphere became a more inefficient insulator for the meager heating from the distant Sun. There appears to be very large amounts of water frozen into the regolith just bellow the surface. These areas may exist even at the Martian Equator.
In 2004 the Mars Express orbiter detected spectral evidence of water in the south pole's ice cap and the surrounding area, which ruled out the possibility that the southern ice cap consisted of only carbon dioxide ice.[3]
In 2005, Mars Express located an area of solid water ice near the north pole.
The Phoenix lander confirmed in 2008 that water ice is not limited to the extreme polar regions.[4]
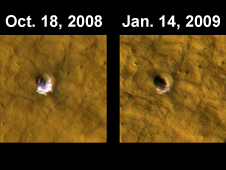
Photos from the Mars Reconnaissance Orbiter show frozen water just below the Martian surface (see photograph). Surprisingly the location is far away from the poles (43.28 degrees north latitude, 164.22 degrees east longitude), which raises the hope of large amounts of water all over the planet. [5]
Abundance
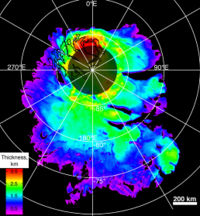
On March 15, 2007, Mars Express' mission control released more news of extensive frozen water discovered at the Martian south pole. These new and highly accurate measurements predict that if the ice were to be melted, the whole planet would be covered in a liquid layer 11 meters deep.[6] Although it has been known for many years that the poles have an abundance of ice, it has never been measured to this degree of accuracy. The data comes from the Mars Advanced Radar for Subsurface and Ionospheric Sounding (MARSIS) currently mapping the north pole to gain a better understanding of how much frozen water may be contained there. MARSIS can probe over 2 miles below the Martian surface and has found extensive layered deposits of ice.
Current liquid water
Future manned exploration on Mars will require a source of water whether it is in the form of ice or sub-surface aquifers. The Mars Express orbiter has uncovered some confusing measurements suggesting there may be liquid water accompanying all that ice. MARSIS bounced back data suggesting at least 90% of the layered deposits under the polar cap are indeed supplies of ice, but a thin layer resembling liquid water is also evident. It is hard to understand the existence of liquid water at the extremely low temperatures predicted. Perhaps high pressures or small geological processes may explain these observations. Another orbiter, NASA's Mars Global Surveyor, has also returned some exciting new evidence for the existence of new flows of liquid water on the Martian surface away from the frozen poles.
| ? |
"What pressures are required to keep water in a liquid phase at temperatures as low as that on the surface of Mars? - Ioneill" |
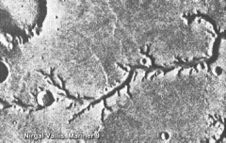
The Mars Global Surveyor arrived at the Red Planet on September 11, 1997 and returned a decade of data on the evolution of the planet before it was lost in November 2006 through energy loss. It was Mars' longest operational artificial satellite. The Mars Orbiter Camera (MOC) onboard revealed new deposits possibly carried as sediment by flowing water in two locations in the past 7 years (press release dated December 6, 2006)[7]. In images taken in August 1999 and September 2006 of the same location (Centauri Montes Region), a bright deposit measuring several hundred meters long is evident in the 2006 image but not in the 1999 image. A similar feature was observed at a different location from 2001 to 2005 at Terra Sirenum. It is worth noting that both locations are in equatorial regions, not usually associated with ice or liquid water. This suggests liquid water remains a characteristic of the Mars landscape, if only sporadically. These discoveries have increased the enthusiasm for the search for microbial life, but the implications for manned exploration are huge. If there are pockets of liquid water just below the surface, Mars may yet be able to provide our future pioneers with natural springs more familiar on Earth.

However, surface water on Mars is short-lived. The Martian atmosphere is very thin (a pressure of 7 millibars, <1% that of Earth's thick atmosphere) and cold (an average global temperature of -55°C or -67F), these two factors deny any long-term existence of liquid water. Surface liquid water will quickly freeze and sublime into the atmosphere, bypassing the liquid phase. This phase transition for water on Mars is much like the phase transition for liquid carbon dioxide on Earth when it is released from a CO2 fire extinguisher to produce dry ice snow and CO2 gas. The phase transition for H2O on the surface of Mars occurs below the "triple point" on the phase diagram so the recent observations of sediment on the surface will have been deposited very quickly by short lived "spurts" of water. Just how short-lived these spurts of water are it is unknown, but a significant volume must have created a formidable river to carry sediment several hundred meters.
At times, the humidity of the Martian atmosphere can reach 100% (at Mars' temperature and pressure). If the temperature was high, salty brines could last several minutes on the Martian surface.
Although there may be other explanations for these long "channels" of sediment, such as rock slides or wind-blown sand features, the appearance of the deposits seem very water-like. Michael Malin of Malin Space Science Systems, a mission scientist for the MOC says, "The shapes of these deposits are what you would expect to see if the material were carried by flowing water... they have finger-like branches at the downhill end and easily diverted around small obstacles".[8] It is also possible that other liquids such as 1,2-butanediol, 1,3-butanediol, 1,2-propanediol, 1,3-propanediol, ethylene glycol and related liquids could be responsible for fluid flow features on Mars. While such liquids would be relatively more rare than water, the resistance to freezing of such liquids and mixtures of such liquids with water would allow them to cause fluid flow effects where pure liquid water is impossible.
Water production
Water production on Mars for settlement use or for exploration uses can take many forms. As a prime In-situ resource the presence of water on Mars is one of its main attractions. Further exploration is needed to determine if the water is available in a relatively pure form, or if it will contain salts and other chemical contaminants requiring water treatment.
Atmosphere
The Martian atmosphere contains water vapour (which on occasion can reach 100% humidity). However, it is normally dry and very thin, which makes extracting water slow and energy intensive. With a device similar to an air dehumidifier the production of water should be feasible all over the planet's surface. An experimental setup is necessary to find out all about the viability of this approach.
"The University of Washington has designed an in situ resource utilization system to provide water to a life support system in the laboratory module of the NASA Reference Mission to Mars. This system, the Water Vapor Adsorption Reactor (WAVAR), extracts water vapor from the Martian atmosphere by adsorption in a bed of type 3A zeolite molecular sieve. The zeolite 3A adsorbs the water vapor until nearly saturated and is then heated within a sealed chamber by microwave radiation to drive off the water for collection. The water vapor flows to a condenser where it freezes and is later liquefied for use in the life support system. In the NASA Reference Mission, water, methane, and oxygen are produced for life support and propulsion via the Sabatier/Electrolysis process from seed hydrogen brought from Earth and Martian atmospheric carbon dioxide. In order for the WAVAR system to be compatible with the NASA Reference Mission, its mass must be less than that of the seed hydrogen and cryogenic tanks apportioned for life support in the Sabatier/Electrolysis process. The WAVAR system is designed for atmospheric conditions observed by the Viking missions, which measured an average global atmospheric water vapor concentration of approx. 2 x 10-6kg/cubic meter. WAVAR performance is analyzed taking into consideration hourly and daily fluctuations in Martian ambient temperature and the corresponding effects on zeolite performance." [9]
Caves
Since the discovery of caves scientists believe in the possibility of water ice on the ground of the caves. Water ice is abundant under the ground at least near the poles, and probably elsewhere too.
Glaciers
The Mars Reconnaissance Orbiter has found evidence of glaciers covered in regolith.[10] Radar reflection data indicates that these are not Rock Glaciers that have been previously suspected on Mars, but instead are thick glacial ice covered in a thin layer of debris. The buried glaciers lie in the Hellas Basin region of Mars' southern hemisphere with similar aprons detected extending from cliffs in the northern hemisphere.
Regolith
Water is present in the martian regolith both as ice and as hydrated minerals. In addition, a recent paper has shown that a huge amount of water has been absorbed by rocks. On Earth, plate Tectonics takes these hydrated minerals and melts them, where water can return to the surface via vulcanism. On Mars the water remains in these minerals. [11]
One way to obtain water from regolith is to cover a spot with a clear cover, heat it with focused sunlight (mirrors), and let the water vapor condense on the cover. Collect the condensate on the edges of the cover. At ground level the Martian atmosphere has a pressure of 6.518 millibars or 0.095 psi as compared to the Earth's sea level atmospheric pressure of 14.7 psi. [12] Boiling point of water at 6.518 millibars: 1.5 degC, 34.7 degF. [13]
"Excavated regolith can be processed by crushing, dry-grinding, and running it through a continuous feed electrically heated rotary kiln, heating to temperatures above 500˚C to decompose perchlorates, [14][15][16]
[Ca(H2O)4](ClO4)2 -> Ca(ClO4)2 + 4H2O
oxides, sulfates, carbonates, and nitrates driving off gases and water vapor. The gases are processed through aseries of PSA equipment. The remaining regolith can be sifted and sorted, using various ore processing techniques, such as magnetic separation for iron rich minerals, and sifting to produce aggregate mixtures suitable for producing 3D printed sulfur concrete."
Polar regions
The martian polar regions have extensive ice caps as well as ice filled craters, such as the Korolev crater, that could serve as water sources. As the poles are usually extremely cold, sources closer to the equator would be more useful for future martian settlements. Note that permafrost has been detected at all latitudes, but above 30 degrees latitude, surface ice is found fairly often.
Uses
Drinking water
The human metabolism requires a regular intake of fresh water. Pure liquid water is non-existent on Mars but there is abundant frozen water and hydrated minerals. Since getting liquid water for use will require some industrial effort, the recycling of all excretion would provide advantages in reducing water use. There are two ways: Wastewater can be treated, which is partially done on the ISS already. Alternatively, the water can be kept in a nearly natural cycle, using parts of the greenhouses for biological wastewater treatment.
The concentration of deuterium in Martian hydrogen and thus in Martian water is between five and thirteen ten-thousandth-parts.[17] or about six times the relative abundance on Earth. Still this concentration is far from the 25% of the body's content of hydrogen that would need to be substituted by deuterium before there are any serious health effects. Deuterium is not a cumulative poison. At about one thousandth part of the hydrogen in Martian water, deuterium would be excreted as fast as it is consumed and would not be a health problem.
Industrial processes
Many industrial processes considered for a Mars settlement, in particular the production of methanol and methane and other hydrocarbons require hydrogen. This can be obtained by electrolysis or via thermolysis such as the Sulfur/Iodine cycle[18] or the Zinc/Sulfur/Iodine cycle[19]. In electrolysis or basic thermolysis, [[Oxygen O2]] is produced as a byproduct. In the Zinc/Sulfur/Iodine cycle, CO2 and H2O are inputs and the outputs are CO and H2. CO is industrially useful in the production of methanol and other hydrocarbons, as well as in mineral refining via the Mond process. The H2 can also be combined with atmospheric N2 using a Haber reactor to produce ammonia.
Most other processes require the use of significant quantities of water as a solvent for reagents such as acids or ammonia or a a coolant for high temperature systems.
Power sources which rely on heat engines (such as nuclear power) require a heat sink to provide the heat differential required for the engine to run. Water or Ice make good materials for this heat sink as they are dense and have high thermal mass. The ice of Korolev Crater has been suggested as a potential heat sink sufficient to provide for colony scale power generation.
Deuterium from martian water may provide a source of fusion fuel for future energy production. Note that deuterium is about six times more concentrated on Mars than on Earth, and may form a viable export.
Methanol and methane may be used to feed methanotrophs to produce food or other biologically produced industrial materials.
See Also
- Martian features that are signs of water ice
- Sublimation
- Water infrastructure and waste water treatment
- "Mars: A Warmer Wetter Planet", by Jeffrey S. Kargel, ISBN 1-85233-568-8. This important book collected the evidence of water on Mars, and put to rest the idea that Mars had always been a dry planet.
External links
- Wikipedia page on water
- Searching for water with the Mars Express MARSIS instrument.
- Wikipedia page on water on Mars
- The Evolution of Water on Mars
- James Wray - The Search for Water and Life on Mars (and Beyond) (November 15, 2018)
- Mars: Ancient Water, Present Day Ice
- Water on Mars and the Potential for Martian Life
- Oceans and Life on Mars
References
- ↑ Michael P. Lamb, John P. Grotzinger, John B. Southard, Nicholas J. Tosca, 2012. "Were Aqueous Ripples on Mars Formed by Flowing Brines?", Sedimentary Geology of Mars, John P. Grotzinger, Ralph E. Milliken. https://doi.org/10.2110/pec.12.102.0139
- ↑ The European Space Agency. September 1 2019. Mars Express science highlights: #1. Hydrated minerals – evidence of liquid water on Mars. https://sci.esa.int/web/mars-express/-/51821-1-hydrated-minerals-ndash-evidence-of-liquid-water-on-mars
- ↑ European Space Agency. March 17, 2004. Water at Martian south pole. https://www.esa.int/Science_Exploration/Space_Science/Mars_Express/Water_at_Martian_south_pole
- ↑ Smith, P., et al. 2009. H2O at the Phoenix Landing Site. Science: 325, 58-61.
- ↑ Mars Reconnaissance Orbiter Sees Ice Exposed by Meteor Impact
- ↑ The European Space Agency. September 1 2019. Mars Express science highlights: #4. Probing the polar regions. https://sci.esa.int/web/mars-express/-/51824-4-probing-the-polar-regions
- ↑ NASA Press Release: NASA Images Suggest Water Still Flows in Brief Spurts on Mars
- ↑ NASA Press Release: NASA Images Suggest Water Still Flows in Brief Spurts on Mars
- ↑ Sergio Adan-Plaza, Kirsten Carpenter, Laila Elias, Rob Grover, Mark Hilstad, Chris Hoffman, Matt Scheider, & Adam Bruckner. (1998). Extraction of Atmospheric Water on Mars for the Mars Reference Mission. Lpi.usra.edu. Retrieved 15 November 2021, from https://www.lpi.usra.edu/publications/reports/CB-955/washington.pdf.
- ↑ http://www.nasa.gov/home/hqnews/2008/nov/HQ_08-304_MRO_BuriedGlaciers.html
- ↑ https://science.sciencemag.org/content/early/2021/03/15/science.abc7717 - Long term drying of Mars by sequestration of Ocean-scale volumes of water in the crust
- ↑ Mars. Mars.nasa.gov. (1997). Retrieved 15 November 2021, from https://mars.nasa.gov/MPF/mpf/realtime/mars2.html.
- ↑ Water - Boiling Points at Vacuum Pressure. Engineeringtoolbox.com. (2021). Retrieved 15 November 2021, from https://www.engineeringtoolbox.com/water-evacuation-pressure-temperature-d_1686.html.
- ↑ James D. Little (2019). 3: Aeneas Complex: A Plan For A Sustainable, Permanent 1000 Person Settlement On Mars in the book Mars colonies: Plans for Settling the Red Planet (p. 58).
- ↑ Marvin, G., Woolaver, L., Thermal Decomposition of Perchlorates, Industrial & Engineering Chemistry Analytical Edition, 1945, Vol. 17, Iss. 8, pp. 474-476.
- ↑ Bruck. A., Sutter, B., Ming, D., Mahaffy, P., Thermal Decomposition of Calcium Perchlorate/Iron-mineral Mixtures: Implications of the Evolved Oxygen from the Rocknest Eolian Deposit in Gale Crater, Mars., 45th Lunar and Planetary Science Conference, Mar. 2014.
- ↑ abstract in Science
- ↑ https://doi.org/10.1016/j.ijhydene.2006.05.013
- ↑ https://doi.org/10.1016/j.ijhydene.2015.11.049